Back
Poster, Podium & Video Sessions
Moderated Poster
MP56: Bladder Cancer: Invasive V
MP56-20: Surrogate endpoints as predictors of survival in metastatic urothelial cancer trials
Monday, May 16, 2022
10:30 AM – 11:45 AM
Location: Room 228
Fady Ghali*, Seattle, WA, Devin Patel, Denver, CO, Teresa Jewell, John Gore, Jonathan Wright, Seattle, WA

Fady Ghali, MD
MD
University of Washington
Poster Presenter(s)
Introduction: Surrogate endpoints such as progression-free survival (PFS) and objective response rate (ORR) are frequently used in clinical trials. The relationship between surrogate endpoints and overall survival (OS) is not well described in metastatic urothelial carcinoma (UC). To address this gap in knowledge, we evaluated trial-level data to assess the statistical relationship between surrogate endpoints and OS.
Methods: We systematically reviewed Phase II/III trials in metastatic UC that were written in English, have at least two treatment arms, and report median PFS and/or ORR and OS. Data on publication year, class of drug/intervention, presence of industry funding, and median PFS/ORR and OS were recorded. Linear regression was performed, and coefficient of determination (R2) and surrogate threshold effect (STE) estimate was determined between PFS/ORR and OS.
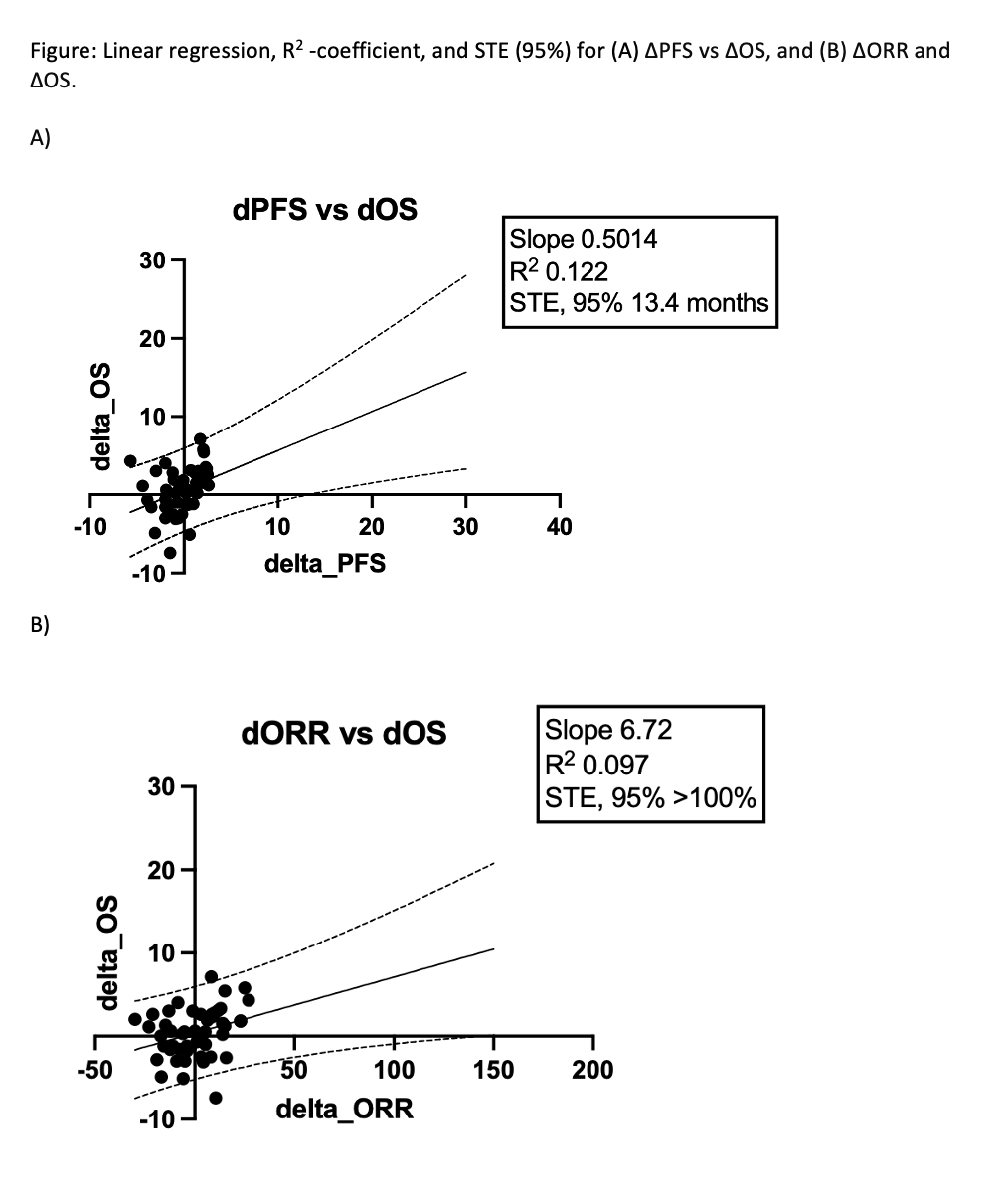
Results: From 3254 trials that resulted from our initial search terms, 57 studies and 62 comparisons met our inclusion criteria and were analyzed. The median year of publication was 2016 [IQR 2006, 2020]; the average number of participants per study was 128 [IQR 85, 370]; and the median follow-up was 25.30 [IQR 16.40, 41.20] months. Median surrogate endpoint changes were ?PFS (PFS of treatment arm – PFS control arm) of 0.20 months [IQR -1.50, 1.40] and ?ORR of 2.50% [IQR -8.93%, 10.40%]. Median ?OS was 0.60 months [IQR -1.20, 2.60]. Linear regression demonstrated a wide scatter between PFS/ORR and OS, and R2 analysis found R2 of 0.122 between ?PFS and ?OS, and 0.097 between ?ORR and ?OS. STE analysis determined that a ?PFS of 13.4 months was sufficient to provide 95% confidence of ?OS >0; no trials met this threshold. STE for ?ORR was >100%; no trials met this infeasible threshold.
Conclusions: The surrogate endpoints PFS and ORR are weakly correlated with OS in metastatic UC. Trials demonstrating a 13.4-month improvement in PFS can claim an OS benefit with 95% confidence, while ORR could not provide this confidence. Caution should be exercised when using surrogate endpoints in lieu of OS in metastatic UC trials.
Source of Funding: None
Methods: We systematically reviewed Phase II/III trials in metastatic UC that were written in English, have at least two treatment arms, and report median PFS and/or ORR and OS. Data on publication year, class of drug/intervention, presence of industry funding, and median PFS/ORR and OS were recorded. Linear regression was performed, and coefficient of determination (R2) and surrogate threshold effect (STE) estimate was determined between PFS/ORR and OS.
Results: From 3254 trials that resulted from our initial search terms, 57 studies and 62 comparisons met our inclusion criteria and were analyzed. The median year of publication was 2016 [IQR 2006, 2020]; the average number of participants per study was 128 [IQR 85, 370]; and the median follow-up was 25.30 [IQR 16.40, 41.20] months. Median surrogate endpoint changes were ?PFS (PFS of treatment arm – PFS control arm) of 0.20 months [IQR -1.50, 1.40] and ?ORR of 2.50% [IQR -8.93%, 10.40%]. Median ?OS was 0.60 months [IQR -1.20, 2.60]. Linear regression demonstrated a wide scatter between PFS/ORR and OS, and R2 analysis found R2 of 0.122 between ?PFS and ?OS, and 0.097 between ?ORR and ?OS. STE analysis determined that a ?PFS of 13.4 months was sufficient to provide 95% confidence of ?OS >0; no trials met this threshold. STE for ?ORR was >100%; no trials met this infeasible threshold.
Conclusions: The surrogate endpoints PFS and ORR are weakly correlated with OS in metastatic UC. Trials demonstrating a 13.4-month improvement in PFS can claim an OS benefit with 95% confidence, while ORR could not provide this confidence. Caution should be exercised when using surrogate endpoints in lieu of OS in metastatic UC trials.
Source of Funding: None


.jpg)
.jpg)