Back
Poster, Podium & Video Sessions
Moderated Poster
MP59: Bladder Cancer: Non-Invasive III
MP59-14: Cross-Sectional Study Assessing Utilization Patterns of Combination Intravesical Gemcitabine and Docetaxel in the Management of Bladder Cancer
Monday, May 16, 2022
1:00 PM – 2:15 PM
Location: Room 225
Andrew Gabrielson*, Sunil Patel, Noah Hahn, Baltimore, MD, Angela Smith, Chapel Hill, NC, Eugene Pietzak, New York, NY, Peter Black, Vancouver, Canada, Trinity Bivalacqua, Philadelphia, PA, Max Kates, Baltimore, MD

Andrew Gabrielson, MD (he/him/his)
Johns Hopkins University School of Medicine
Poster Presenter(s)
Introduction: Intravesical bacillus-calmette Guerin (BCG) is a mainstay treatment of non-muscle invasive bladder cancer (NMIBC), however BCG therapy is fraught with frequent drug shortages and its scarcity has prompted investigation into alternative agents. Intravesical gemcitabine and docetaxel (GemDoce) is a safe therapy for BCG-unresponsive NMIBC and BCG naïve NMIBC in times of BCG shortage with efficacy approaching BCG in retrospective series. We sought to assess utilization and barriers to administration of GemDoce to inform accrual for a phase 3 randomized trial.
Methods: A cross-sectional, web-based survey was distributed via the Society of Urologic Oncologists (SUO) and Canadian Urologic Oncology Group (CUOG) mailing lists using Google Forms. Provider prescribing patterns including the preferred setting of GemDoce use, annual prescribing rate, and description of barriers to administration of GemDoce were assessed.
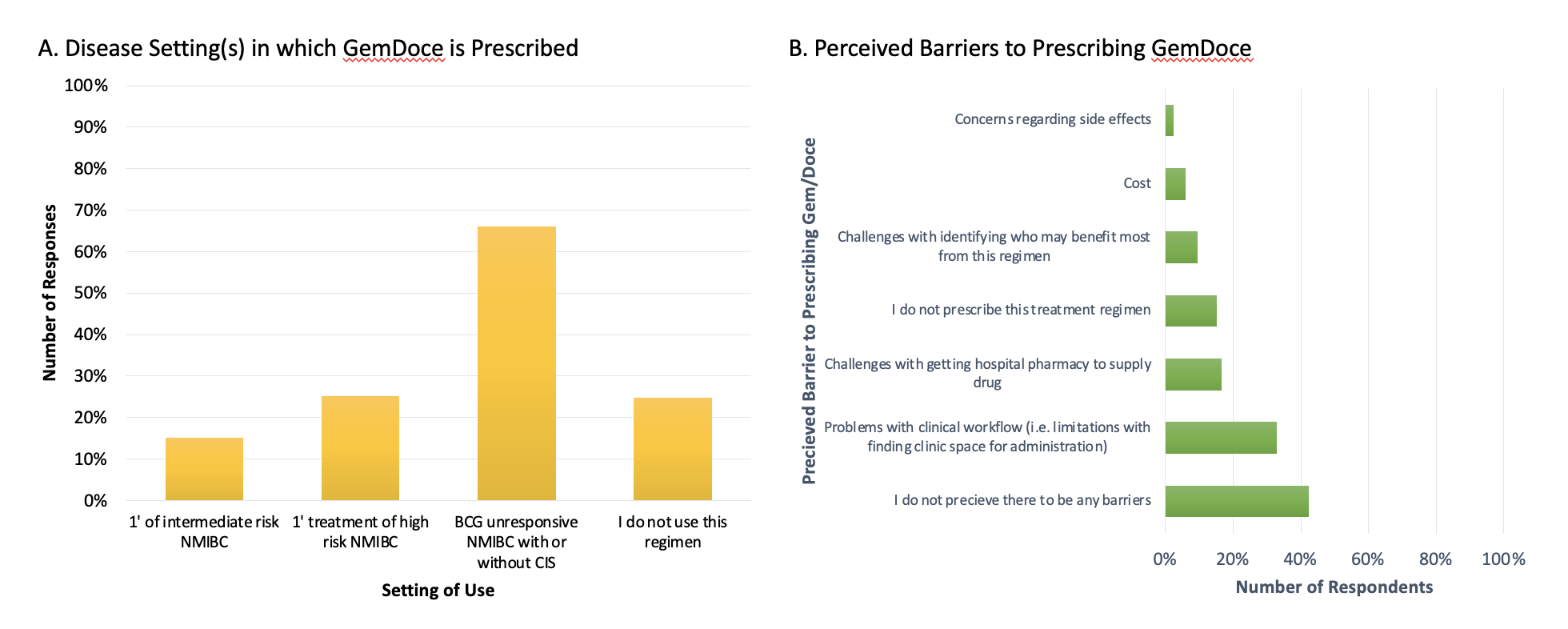
Results: The survey was distributed to 982 addresses (661 report bladder cancer in their practice). A total of 198 participants completed the survey (30% response rate for target audience), of which 141 (71%) endorsed prescribing GemDoce over the last 12 months. The most common indication was BCG-unresponsive NMIBC +/- CIS (67%), followed by primary treatment of high-risk NMIBC (25%) and primary treatment of intermediate-risk NMIBC (15%). Based on conservative estimates of participant responses, at least 1,300 patients within the practices of the surveyed urologists are being prescribed GemDoce for NMIBC on an annual basis. Notably, 44% of participants endorsed at least one barrier to prescribing or administering GemDoce at their institution. Common barriers included problems with clinical workflow (i.e. clinic space to administer drug), challenges with hospital pharmacies supplying the drug, challenges with identifying who would benefit most from this regimen, and cost (Figure 1).
Conclusions: Results from this cross-sectional web-based survey demonstrate that GemDoce is being widely utilized among urologic oncologists for several disease states of NMIBC. Several barriers exist that may hinder use of GemDoce and future clinical trial designs will need to account for these potential challenges in clinical workflow.
Source of Funding: N/A
Methods: A cross-sectional, web-based survey was distributed via the Society of Urologic Oncologists (SUO) and Canadian Urologic Oncology Group (CUOG) mailing lists using Google Forms. Provider prescribing patterns including the preferred setting of GemDoce use, annual prescribing rate, and description of barriers to administration of GemDoce were assessed.
Results: The survey was distributed to 982 addresses (661 report bladder cancer in their practice). A total of 198 participants completed the survey (30% response rate for target audience), of which 141 (71%) endorsed prescribing GemDoce over the last 12 months. The most common indication was BCG-unresponsive NMIBC +/- CIS (67%), followed by primary treatment of high-risk NMIBC (25%) and primary treatment of intermediate-risk NMIBC (15%). Based on conservative estimates of participant responses, at least 1,300 patients within the practices of the surveyed urologists are being prescribed GemDoce for NMIBC on an annual basis. Notably, 44% of participants endorsed at least one barrier to prescribing or administering GemDoce at their institution. Common barriers included problems with clinical workflow (i.e. clinic space to administer drug), challenges with hospital pharmacies supplying the drug, challenges with identifying who would benefit most from this regimen, and cost (Figure 1).
Conclusions: Results from this cross-sectional web-based survey demonstrate that GemDoce is being widely utilized among urologic oncologists for several disease states of NMIBC. Several barriers exist that may hinder use of GemDoce and future clinical trial designs will need to account for these potential challenges in clinical workflow.
Source of Funding: N/A


.jpg)
.jpg)