Back
Poster, Podium & Video Sessions
Moderated Poster
MP59: Bladder Cancer: Non-Invasive III
MP59-16: Sequential Intravesical Valrubicin and Docetaxel for the treatment of Non-Muscle Invasive Bladder Cancer
Monday, May 16, 2022
1:00 PM – 2:15 PM
Location: Room 225
Ian M. McElree*, Vignesh T. Packiam, Ryan L. Steinberg, Sarah L. Mott, Paul T. Gellhaus, Kenneth G. Nepple, Michael A. O'Donnell, Iowa City, IA

Ian Mitchell Mcelree, BS, MS
The University of Iowa
Poster Presenter(s)
Introduction: Intravesical gemcitabine-docetaxel (Gem/Doce) has emerged as an efficacious and well-tolerated therapy for NMIBC. However, effective rescue therapies are needed for subsequent recurrences, particularly when cystectomy is refused or precluded. Current FDA-approved agents for BCG failures all show <20% 1 year response. We report our experience with sequential intravesical valrubicin and docetaxel (Val/Doce) as a rescue therapy for NMIBC.
Methods: We retrospectively identified all patients with recurrent NMIBC who were treated with Val/Doce between April 2013 and June 2021. Patients were treated with weekly sequential intravesical instillations of 800 mg valrubicin and 37.5 mg docetaxel for 6 weeks. Monthly maintenance of 2 years was initiated if disease free at first follow up. Analysis was stratified by low grade (LG) and high grade (HG) disease. The primary outcomes were recurrence-free survival (RFS) and HG RFS. Progression was defined as development of muscle invasive or metastatic disease or cystectomy. Survival was assessed using the Kaplan-Meier method, indexed from start of Val/Doce induction. Surveillance was performed according to AUA guidelines.
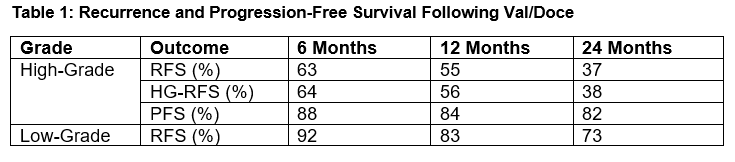
Results: Seventy-five patients with median follow-up of 21 (IQR: 13-37) months were included in the analysis. Previous intravesical failures included Gem/Doce (95%), BCG (72%), and both BCG and Gem/Doce (68%). Twelve patients with recurrent LG disease showed a 73% 2-year RFS with no HG recurrences (Table 1). Sixty-three patients had recurrent HG disease with a median of 2 prior induction courses. The 1 and 2-year HG RFS were 56% and 38%, respectively. Forty-two (56%) patients had CIS present. RFS was similar for those with and without CIS (p=0.63). Progression occurred in 12 (16%) patients. Ten patients (16%) underwent cystectomy and 2 (3%) died of metastatic bladder cancer. Overall, cancer-specific, and cystectomy-free survival were 88%, 96%, and 85% at 24 months, respectively. Common side effects were bladder spasms (24%), urinary frequency (13%), and dysuria (11%). Three patients could not tolerate a full induction course.
Conclusions: In a heavily pre-treated population, Val/Doce showed promising efficacy as a rescue treatment for patients with recurrent NMIBC. Further prospective evaluation of Val/Doce is needed.
Source of Funding: This work was supported by the John & Carol Walter Family Foundation and the Carver College of Medicine.
Methods: We retrospectively identified all patients with recurrent NMIBC who were treated with Val/Doce between April 2013 and June 2021. Patients were treated with weekly sequential intravesical instillations of 800 mg valrubicin and 37.5 mg docetaxel for 6 weeks. Monthly maintenance of 2 years was initiated if disease free at first follow up. Analysis was stratified by low grade (LG) and high grade (HG) disease. The primary outcomes were recurrence-free survival (RFS) and HG RFS. Progression was defined as development of muscle invasive or metastatic disease or cystectomy. Survival was assessed using the Kaplan-Meier method, indexed from start of Val/Doce induction. Surveillance was performed according to AUA guidelines.
Results: Seventy-five patients with median follow-up of 21 (IQR: 13-37) months were included in the analysis. Previous intravesical failures included Gem/Doce (95%), BCG (72%), and both BCG and Gem/Doce (68%). Twelve patients with recurrent LG disease showed a 73% 2-year RFS with no HG recurrences (Table 1). Sixty-three patients had recurrent HG disease with a median of 2 prior induction courses. The 1 and 2-year HG RFS were 56% and 38%, respectively. Forty-two (56%) patients had CIS present. RFS was similar for those with and without CIS (p=0.63). Progression occurred in 12 (16%) patients. Ten patients (16%) underwent cystectomy and 2 (3%) died of metastatic bladder cancer. Overall, cancer-specific, and cystectomy-free survival were 88%, 96%, and 85% at 24 months, respectively. Common side effects were bladder spasms (24%), urinary frequency (13%), and dysuria (11%). Three patients could not tolerate a full induction course.
Conclusions: In a heavily pre-treated population, Val/Doce showed promising efficacy as a rescue treatment for patients with recurrent NMIBC. Further prospective evaluation of Val/Doce is needed.
Source of Funding: This work was supported by the John & Carol Walter Family Foundation and the Carver College of Medicine.

.jpg)
.jpg)