Back
Poster, Podium & Video Sessions
Podium
PD01: Kidney Cancer: Basic Research & Pathophysiology I
PD01-05: MiR-182-5p Inhibits the Tumorigenesis of clear cell renal cell carcinoma by Repressing UBE2T
Friday, May 13, 2022
7:40 AM – 7:50 AM
Location: Room 252
Wu Yucai*, Gong Yanqing, Li Xuesong, Beijing, China, People's Republic of, Zhou Liqun, Beijing , China, People's Republic of
- WY
Podium Presenter(s)
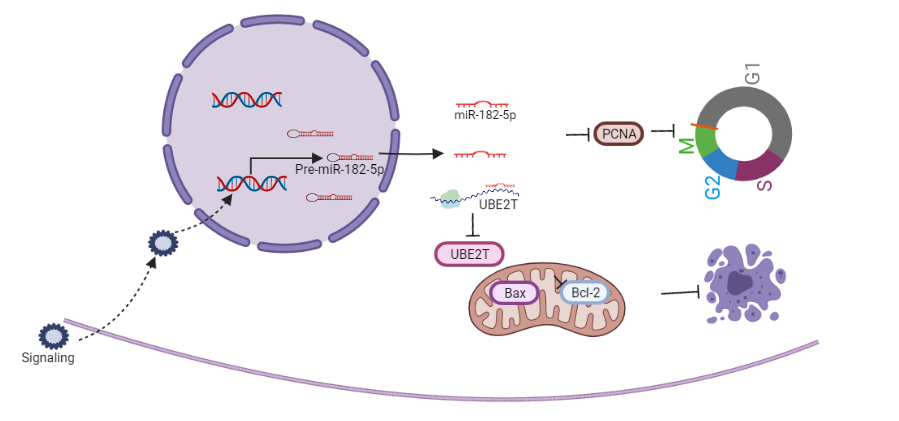
Introduction: Ubiquitin-conjugating enzyme E2T (UBE2T), a member of the E2 family, has been reported to be overexpressed in certain tumor types and to have an important role in the Fanconi anemia pathway. However, the role of UBE2T in clear cell renal cell carcinoma (ccRCC) has not been clarified. MicroRNAs (miRNAs) participate in tumorigenesis by binding to genes and proteins that regulate cell proliferation or cell apoptosis. The aim of this study was to determine the role of UBE2T and the relationship between miR-182-5p and UBE2T in ccRCC.
Methods: UBE2T expression levels were detected by real-time quantitative PCR (RT-qPCR) and western blot in ccRCC cell lines and tissues. Protein expression of UBE2T was assessed in a total of 93 ccRCC patients from Peking University First Hospital (PKU) by immunohistochemistry (IHC). The effects of UBE2T knockdown/overexpression on the proliferation, invasion and metastasis of ccRCC cells were assessed by MTS assays, wound healing assays, transwell invasion assays and ?ow cytometry. The effects of in vivo treatment were evaluated through xenograft experiments. The relationship between miR-182-5p and UBE2T was verified by dual luciferase reporter gene assay.
Results: UBE2T was highly expressed in ccRCC cells and tissues. High expression of UBE2T was positively correlated with advanced pathological stage, histological grade, maximum diameter and distant metastasis of the tumor. Multivariate analysis revealed that the UBE2T expression was an independent risk factor for overall survival (OS) and recurrence-free survival (RFS) in patients with ccRCC. Knockdown of UBE2T significantly suppressed proliferation, migration and invasion of RCC. Flow cytometry analysis showed that knocking down UBE2T could promote RCC cell cycle arrest at G2/M phase and increase cell apoptosis. A xenograft model also confirmed that suppression of UBE2T significantly delayed tumor formation and growth in vivo. In addition, miR-182-5p inhibited the expression of UBE2T protein through targeting UBE2T mRNA, and then inhibited the proliferation, migration and invasion of ccRCC.
Conclusions: Our research reveals that miR-182-5p and UBE2T probably play a critical role in ccRCC progression, and they may be the potential therapeutic targets for ccRCC.
Source of Funding: The present study was funded by the National Key R&D Program of China (grant no. 2019YFA09006001) and the National Natural Science Foundation of China (grant nos. 81772703 and 81972380).
Methods: UBE2T expression levels were detected by real-time quantitative PCR (RT-qPCR) and western blot in ccRCC cell lines and tissues. Protein expression of UBE2T was assessed in a total of 93 ccRCC patients from Peking University First Hospital (PKU) by immunohistochemistry (IHC). The effects of UBE2T knockdown/overexpression on the proliferation, invasion and metastasis of ccRCC cells were assessed by MTS assays, wound healing assays, transwell invasion assays and ?ow cytometry. The effects of in vivo treatment were evaluated through xenograft experiments. The relationship between miR-182-5p and UBE2T was verified by dual luciferase reporter gene assay.
Results: UBE2T was highly expressed in ccRCC cells and tissues. High expression of UBE2T was positively correlated with advanced pathological stage, histological grade, maximum diameter and distant metastasis of the tumor. Multivariate analysis revealed that the UBE2T expression was an independent risk factor for overall survival (OS) and recurrence-free survival (RFS) in patients with ccRCC. Knockdown of UBE2T significantly suppressed proliferation, migration and invasion of RCC. Flow cytometry analysis showed that knocking down UBE2T could promote RCC cell cycle arrest at G2/M phase and increase cell apoptosis. A xenograft model also confirmed that suppression of UBE2T significantly delayed tumor formation and growth in vivo. In addition, miR-182-5p inhibited the expression of UBE2T protein through targeting UBE2T mRNA, and then inhibited the proliferation, migration and invasion of ccRCC.
Conclusions: Our research reveals that miR-182-5p and UBE2T probably play a critical role in ccRCC progression, and they may be the potential therapeutic targets for ccRCC.
Source of Funding: The present study was funded by the National Key R&D Program of China (grant no. 2019YFA09006001) and the National Natural Science Foundation of China (grant nos. 81772703 and 81972380).


.jpg)
.jpg)