Back
Poster, Podium & Video Sessions
Podium
PD02: Pediatric Urology: Neurogenic Bladder, Reconstruction & DSD
PD02-05: Efficacy and safety of vibegron as adjuvant treatment on refractory neurogenic bladder dysfunction in children with spina bifida
Friday, May 13, 2022
7:40 AM – 7:50 AM
Location: Room 255
KAZUYUKI NISHINAKA*, Naoya Masumori, Sapporo, Japan
- KN
Podium Presenter(s)
Introduction: The aim of this study was to evaluate the efficacy and safety of vibegron with improved selectivity and metabolic stability in comparison with previously disclosed ß3-adrenoceptor agonists for treating antimuscarinic-resistant neurogenic bladder dysfunction in children with spina bifida.
Methods: In this retrospective study, 19 patients below 15 years of age, with antimuscarinic-resistant neurogenic bladder dysfunction due to spina bifida, underwent a video-urodynamic study before and during the prescription of vibegron. The dosage was 12.5, 25 or 50 mg once daily, based on age and body weight, for at least 12 weeks from January 2020 to July 2021, in addition to antimuscarinic therapy. The video-urodynamic study was conducted to evaluate bladder compliance, maximum cystometric bladder capacity, detrusor overactivity, detrusor leak point pressure, and vesicoureteral reflux. This was done subsequently prior to the beginning and after three months of vibegron administration. Patient-reported efficacy and adverse events were measured.
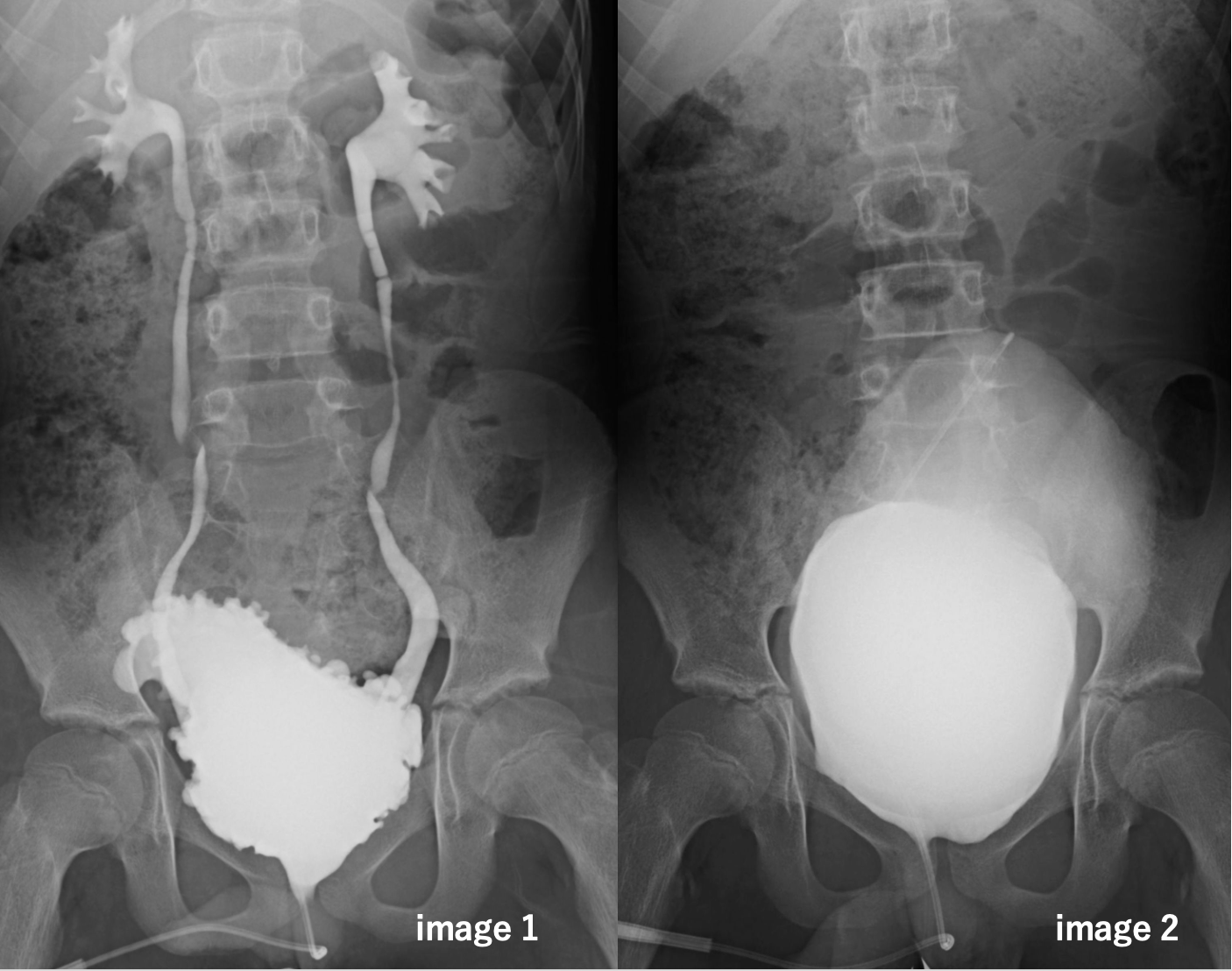
Results: Treatment with vibegron add-on therapy drastically improved bladder compliance and maximum cystometric bladder capacity compared to treatment with antimuscarinic agents alone (6.2; IQR: 5.2–10.4 vs. 28.3; IQR: 22.7–50.7 mL/cmH2O, P < 0.0001; and 178; IQR: 135–235 vs. 450; IQR: 358–502 mL, P<0.0001, respectively). Bladder deformity in 12 patients improved after taking the drug (image 1: before taking vibegron and image 2: after taking vibegron, obtained from the same case). Moreover, vesicoureteral reflux, confirmed in four patients, disappeared post administration. All the 18 patients who were incontinent before remained dry after initiating therapy with vibegron during the daytime. None of the patients showed side effects or discontinued treatment owing to intolerance to vibegron.
Conclusions: Vibegron as adjuvant treatment in children with antimuscarinic-resistant neurogenic bladder dysfunction due to spina bifida showed favorable video-urodynamic efficacy with no apparent adverse events. Vibegron helped to achieve urinary continence in the entire sample population. Vibegron is a favorable treatment option for the treatment of refractory neurogenic bladder dysfunction in children with spina bifida.
Source of Funding: None
Methods: In this retrospective study, 19 patients below 15 years of age, with antimuscarinic-resistant neurogenic bladder dysfunction due to spina bifida, underwent a video-urodynamic study before and during the prescription of vibegron. The dosage was 12.5, 25 or 50 mg once daily, based on age and body weight, for at least 12 weeks from January 2020 to July 2021, in addition to antimuscarinic therapy. The video-urodynamic study was conducted to evaluate bladder compliance, maximum cystometric bladder capacity, detrusor overactivity, detrusor leak point pressure, and vesicoureteral reflux. This was done subsequently prior to the beginning and after three months of vibegron administration. Patient-reported efficacy and adverse events were measured.
Results: Treatment with vibegron add-on therapy drastically improved bladder compliance and maximum cystometric bladder capacity compared to treatment with antimuscarinic agents alone (6.2; IQR: 5.2–10.4 vs. 28.3; IQR: 22.7–50.7 mL/cmH2O, P < 0.0001; and 178; IQR: 135–235 vs. 450; IQR: 358–502 mL, P<0.0001, respectively). Bladder deformity in 12 patients improved after taking the drug (image 1: before taking vibegron and image 2: after taking vibegron, obtained from the same case). Moreover, vesicoureteral reflux, confirmed in four patients, disappeared post administration. All the 18 patients who were incontinent before remained dry after initiating therapy with vibegron during the daytime. None of the patients showed side effects or discontinued treatment owing to intolerance to vibegron.
Conclusions: Vibegron as adjuvant treatment in children with antimuscarinic-resistant neurogenic bladder dysfunction due to spina bifida showed favorable video-urodynamic efficacy with no apparent adverse events. Vibegron helped to achieve urinary continence in the entire sample population. Vibegron is a favorable treatment option for the treatment of refractory neurogenic bladder dysfunction in children with spina bifida.
Source of Funding: None


.jpg)
.jpg)