Back
Poster, Podium & Video Sessions
Podium
PD06: Urodynamics/Lower Urinary Tract Dysfunction/Female Pelvic Medicine: Female Incontinence: Therapy I
PD06-04: A deception study to avoid recall bias for 3 Validated Questionnaires in the office or over the phone for women with or without Urinary Incontinence
Friday, May 13, 2022
10:00 AM – 10:10 AM
Location: Room 255
Meghana Reddy*, Samuel B. Kusin, Alana L. Christie, Philippe E. Zimmern, Dallas, TX
- MR
Podium Presenter(s)
Introduction: With medical practices increasingly using telehealth technology as a modality for care, confirming the validity of non-office administration of questionnaires intended for office use only is increasingly important. We studied three validated questionnaires: Urinary Distress Inventory 6 – Short Form (UDI-6), International Incontinence 7- Short Form (IIQ-7), and one Quality of Life Survey (QoL) and used a deception model to avoid recall bias.
Methods: Following IRB approval, these 3 questionnaires were prospectively administered in women with and without incontinence over the telephone and then again in person at an FPMRS clinic visit about 2 weeks later. A deception protocol was followed where participants were not fully informed of the intent of the study over the phone and then were consented for the study during their office visit after both the phone and in-person questionnaire scores were obtained to minimize any potential recall biases. For incontinent patients, a urinary analysis was obtained to exclude patients with an underlying UTI. Non-English speakers, those with impaired mental competency or on fluid diets were excluded. The study was powered at 80% to enroll 85 women in order to detect a difference between responses at the 0.05 significance level. The telephone and in-person questionnaires scores were compared using a paired T-test analysis.
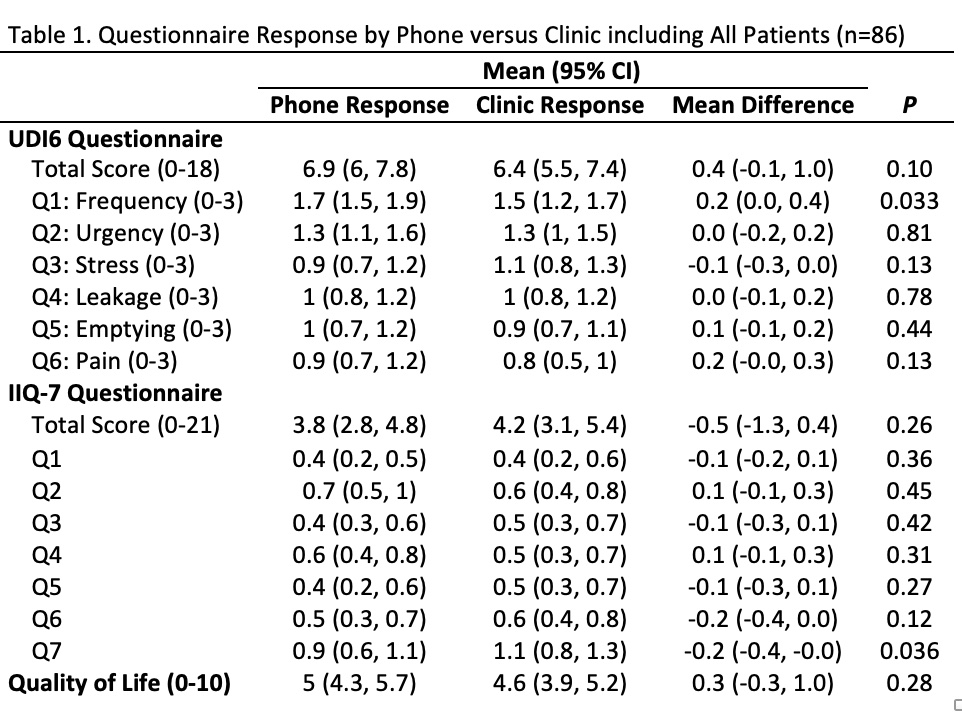
Results: From June to September 2021, 86 women, including 40 incontinent (30-85) and 46 control (30-85), with similar demographic parameters, met all study criteria. Of the 14 questions studied, only two questions, UDI6: Q1 (p = 0.033) and IIQ-7: Q6 (p = 0.036) showed significant differences in responses in patients overall. When only responses from patients with incontinence were compared, only the IIQ-7: Q6 (p = 0.012) showed a significant difference in responses.
Conclusions: The three questionnaire scores were comparable when obtained over the phone or during office visit given that the total scores remained similar and there were minimal differences in responses to individual questions. Women with incontinence, who may otherwise be lost to follow-up or only reachable by telehealth calls, can benefit from the remote administration of these 3 questionnaires.
Source of Funding: N/A
Methods: Following IRB approval, these 3 questionnaires were prospectively administered in women with and without incontinence over the telephone and then again in person at an FPMRS clinic visit about 2 weeks later. A deception protocol was followed where participants were not fully informed of the intent of the study over the phone and then were consented for the study during their office visit after both the phone and in-person questionnaire scores were obtained to minimize any potential recall biases. For incontinent patients, a urinary analysis was obtained to exclude patients with an underlying UTI. Non-English speakers, those with impaired mental competency or on fluid diets were excluded. The study was powered at 80% to enroll 85 women in order to detect a difference between responses at the 0.05 significance level. The telephone and in-person questionnaires scores were compared using a paired T-test analysis.
Results: From June to September 2021, 86 women, including 40 incontinent (30-85) and 46 control (30-85), with similar demographic parameters, met all study criteria. Of the 14 questions studied, only two questions, UDI6: Q1 (p = 0.033) and IIQ-7: Q6 (p = 0.036) showed significant differences in responses in patients overall. When only responses from patients with incontinence were compared, only the IIQ-7: Q6 (p = 0.012) showed a significant difference in responses.
Conclusions: The three questionnaire scores were comparable when obtained over the phone or during office visit given that the total scores remained similar and there were minimal differences in responses to individual questions. Women with incontinence, who may otherwise be lost to follow-up or only reachable by telehealth calls, can benefit from the remote administration of these 3 questionnaires.
Source of Funding: N/A


.jpg)
.jpg)