Back
Poster, Podium & Video Sessions
Podium
PD07: Prostate Cancer: Basic Research & Pathophysiology I
PD07-10: Stromal Androgen Receptor Inhibits Prostate Tumor Progression by Restraining Secretory Luminal Epithelial Cells
Friday, May 13, 2022
11:00 AM – 11:10 AM
Location: Room 245
Zhu Wang*, Yueli Liu, Santa Cruz, CA, Jiawen Wang, Beijing, China, People's Republic of, Corrigan Horton, Joshua Stefanson, Qing Xie, Santa Cruz, CA
- ZW
Podium Presenter(s)
Introduction: Androgen receptor (AR) is expressed in both the prostate epithelium and stroma, and plays cell-type-specific roles in development and prostate cancer (PCa). While it is well established that stromal AR is required for epithelial cell growth in prostate development, how it affects PCa progression remains unclear. Clinically, lower expression of stromal AR is associated with advanced PCa and worse patient outcome. Yet several renal grafting and mouse model studies reported a role for stromal AR in promoting PCa progression. The discrepancy may be attributed to a negligence of developmental vs adult prostate stages in previous studies. The objective of this study is to clarify the role of stromal AR in PCa using rigorous mouse model designs and investigate its mechanism.
Methods: We deleted stromal AR strictly at adult stage by tamoxifen-inducing the Myh11-CreERT2 line in the Hi-Myc and the hormonal carcinogenesis T+E2 mouse models (named strAR- Myc and strAR- T+E, respectively, as experimental groups). Myc and T+E mice with wildtype stroma served as controls. We assessed tumor histology for both models through time by H&E and assigned a score for each sample in a double-blind setting. Experimental and control groups were compared using two-sided t-test. We next performed single cell RNA-seq (scRNA-seq) of T+E and strAR- T+E samples to determine which cell type was altered and used gene set enrichment analysis (GSEA) to determine the affected molecular pathway.
Results: Fig A shows that stromal AR loss consistently resulted in higher H&E scores (more aggressive) in both models. UMAP in Fig B shows that secretory luminal cells were particularly altered by stromal AR loss. GSEA analyses of multiple datasets revealed that the PI3K pathway was potentiated in a subset luminal cells of the strAR- T+E tumor.
Conclusions: Stromal AR normally inhibits PCa progression by restraining the PI3K activity within luminal tumor cells. This implies that conventional androgen deprivation therapy may have unintended negative consequence due to stromal AR loss.
Source of Funding: NIH R01GM116872
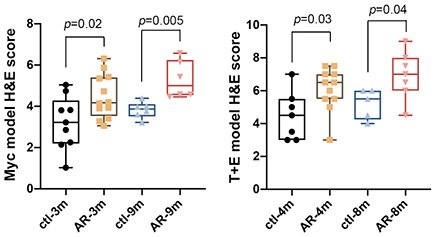
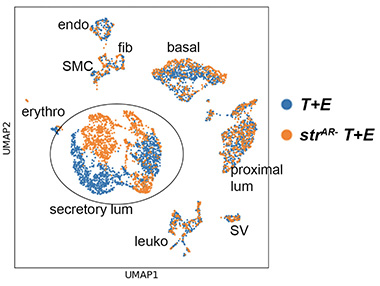
Methods: We deleted stromal AR strictly at adult stage by tamoxifen-inducing the Myh11-CreERT2 line in the Hi-Myc and the hormonal carcinogenesis T+E2 mouse models (named strAR- Myc and strAR- T+E, respectively, as experimental groups). Myc and T+E mice with wildtype stroma served as controls. We assessed tumor histology for both models through time by H&E and assigned a score for each sample in a double-blind setting. Experimental and control groups were compared using two-sided t-test. We next performed single cell RNA-seq (scRNA-seq) of T+E and strAR- T+E samples to determine which cell type was altered and used gene set enrichment analysis (GSEA) to determine the affected molecular pathway.
Results: Fig A shows that stromal AR loss consistently resulted in higher H&E scores (more aggressive) in both models. UMAP in Fig B shows that secretory luminal cells were particularly altered by stromal AR loss. GSEA analyses of multiple datasets revealed that the PI3K pathway was potentiated in a subset luminal cells of the strAR- T+E tumor.
Conclusions: Stromal AR normally inhibits PCa progression by restraining the PI3K activity within luminal tumor cells. This implies that conventional androgen deprivation therapy may have unintended negative consequence due to stromal AR loss.
Source of Funding: NIH R01GM116872



.jpg)
.jpg)