Back
Poster, Podium & Video Sessions
Bladder Cancer: Invasive II
PD10-01: Disease-Free Survival With Longer Follow-Up From the CheckMate 274 Trial of Adjuvant Nivolumab in Patients After Surgery for High-Risk Muscle-Invasive Urothelial Carcinoma
Friday, May 13, 2022
1:00 PM – 1:10 PM
Location: Room 255
Matthew Galsky*, New York, NY, Johannes Alfred Witjes, Nijmegen, Netherlands, Jürgen E. Gschwend, Munich, Germany, Michael Schenker, Craiova, Romania, Begoña P Valderrama, Sevilla, Spain, Yoshihiko Tomita, Niigata, Japan, Aristotelis Bamias, Athens, Greece, Thierry Lebret, Versailles, France, Shahrokh F. Shariat, Vienna, Austria, Se Hoon Park, Seoul, Korea, Republic of, Mads Agerbaek, Aarhus, Denmark, Gautam Jha, Minneapolis, MN, Frank Stenner Stenner, Basel, Switzerland, Sandra Collette, Braine-l’Alleud, Belgium, Keziban Unsal-Kacmaz, Federico Nasroulah, Joshua Zhang, Princeton, NJ, Dean F. Bajorin, New York, NY

Matthew Galsky
Icahn School of Medicine at Mount Sinai
Podium Presenter(s)
Introduction: In the phase 3, randomized, double-blind CheckMate 274 trial of adjuvant nivolumab (NIVO) vs placebo (PBO) in patients (pts) with high-risk muscle-invasive urothelial carcinoma (MIUC) after radical surgery (minimum follow-up in the intent-to-treat [ITT] population 5.9 mo), disease-free survival (DFS) was significantly improved with NIVO vs PBO in ITT pts (HR 0.70; 98.22% CI 0.55-0.90; P<0.001) and in pts with tumor PD-L1 expression =1% (HR 0.55; 98.72% CI 0.35-0.85; P<0.001). We report DFS outcomes with longer follow-up.
Methods: Pts were randomized 1:1 to NIVO 240 mg IV Q2W or PBO for =1 year of adjuvant treatment. Pts had radical surgery within 120 days ± neoadjuvant cisplatin, with evidence of UC at high risk of recurrence per pathologic staging. Primary endpoints were DFS in all randomized (ITT) pts and in pts with PD-L1 =1%. DFS was also evaluated in prespecified subgroups. Non–urothelial tract recurrence-free survival (NUTRFS) in ITT pts and in pts with PD-L1 =1% was a secondary endpoint. Distant metastasis-free survival (DMFS) was an exploratory endpoint.
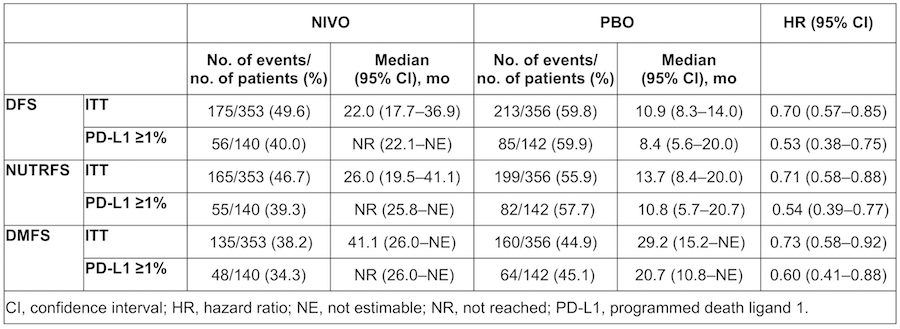
Results: Overall, 353 pts were randomized to NIVO (PD-L1 =1%, n=140) and 356 to PBO (PD-L1 =1%, n=142). With 5 mo additional follow-up, DFS benefit with NIVO vs PBO was maintained in ITT pts (follow-up: minimum 11.0 mo; median 24.4 mo [NIVO] and 22.5 mo [PBO]) and pts with PD-L1 =1% (follow-up: minimum 11.4 mo; median 25.5 mo [NIVO] and 22.4 mo [PBO]; Table). DFS probability at 12 mo was 63.5% with NIVO and 46.9% with PBO in ITT pts. In pts with PD-L1 =1%, DFS probability at 12 mo was 67.6% with NIVO and 46.3% with PBO. Improvement in DFS was observed with NIVO vs PBO for most subgroups analyzed, including age, sex, ECOG PS, nodal status, use of prior cisplatin-based chemotherapy, and PD-L1 status. NUTRFS and DMFS were also improved with NIVO vs PBO in both ITT pts and pts with PD-L1 =1% (Table).
Conclusions: With longer follow-up, NIVO continued to show clinically meaningful improvement in DFS vs PBO for pts with high-risk MIUC, both in ITT pts and in pts with PD-L1 =1%. NUTRFS and DMFS were also improved with NIVO vs with PBO in both pt populations. These results support adjuvant NIVO as a standard of care for high-risk MIUC pts after radical surgery.
Source of Funding: Bristol Myers Squibb
Methods: Pts were randomized 1:1 to NIVO 240 mg IV Q2W or PBO for =1 year of adjuvant treatment. Pts had radical surgery within 120 days ± neoadjuvant cisplatin, with evidence of UC at high risk of recurrence per pathologic staging. Primary endpoints were DFS in all randomized (ITT) pts and in pts with PD-L1 =1%. DFS was also evaluated in prespecified subgroups. Non–urothelial tract recurrence-free survival (NUTRFS) in ITT pts and in pts with PD-L1 =1% was a secondary endpoint. Distant metastasis-free survival (DMFS) was an exploratory endpoint.
Results: Overall, 353 pts were randomized to NIVO (PD-L1 =1%, n=140) and 356 to PBO (PD-L1 =1%, n=142). With 5 mo additional follow-up, DFS benefit with NIVO vs PBO was maintained in ITT pts (follow-up: minimum 11.0 mo; median 24.4 mo [NIVO] and 22.5 mo [PBO]) and pts with PD-L1 =1% (follow-up: minimum 11.4 mo; median 25.5 mo [NIVO] and 22.4 mo [PBO]; Table). DFS probability at 12 mo was 63.5% with NIVO and 46.9% with PBO in ITT pts. In pts with PD-L1 =1%, DFS probability at 12 mo was 67.6% with NIVO and 46.3% with PBO. Improvement in DFS was observed with NIVO vs PBO for most subgroups analyzed, including age, sex, ECOG PS, nodal status, use of prior cisplatin-based chemotherapy, and PD-L1 status. NUTRFS and DMFS were also improved with NIVO vs PBO in both ITT pts and pts with PD-L1 =1% (Table).
Conclusions: With longer follow-up, NIVO continued to show clinically meaningful improvement in DFS vs PBO for pts with high-risk MIUC, both in ITT pts and in pts with PD-L1 =1%. NUTRFS and DMFS were also improved with NIVO vs with PBO in both pt populations. These results support adjuvant NIVO as a standard of care for high-risk MIUC pts after radical surgery.
Source of Funding: Bristol Myers Squibb


.jpg)
.jpg)