Back
Poster, Podium & Video Sessions
Bladder Cancer: Invasive II
PD10-02: Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Long-Term Follow-Up Results From the JAVELIN Bladder 100 Trial
Friday, May 13, 2022
1:10 PM – 1:20 PM
Location: Room 255
Petros Grivas, Seattle, WA, Joaquim Bellmunt*, Boston, MA, Se Hoon Park, Seoul, Korea, Republic of, Eric Voog, Le Mans, France, Claudia Caserta, Terni, Italy, Begoña P. Valderrama, Seville, Spain, Howard Gurney, Sydney, Australia, Yohann Loriot, Villejuif, France, Srikala S. Sridhar, Toronto, Canada, Norihiko Tsuchiya, Yamagata, Japan, Cora N. Sternberg, New York, NY, Jeanny B. Aragon-Ching, Fairfax, VA, Daniel P. Petrylak, New Haven, CT, John A. Blake-Haskins, La Jolla, CA, Robert J. Laliberte, Jing Wang, Cambridge, MA, Nuno Costa, Porto Salvo, Portugal, Thomas Powles, London, United Kingdom

JoaQuin Bellmunt
Harvard Medical School
Podium Presenter(s)
Introduction: The phase 3 JAVELIN Bladder 100 trial (NCT02603432) showed significantly longer overall survival (OS) with avelumab + best supportive care (BSC) vs BSC alone in patients (pts) with advanced urothelial carcinoma (UC) that had not progressed with first-line (1L) platinum-containing chemotherapy. Avelumab 1L maintenance is now considered standard of care in treatment guidelines. We report trial data with =2-years follow-up in all pts (additional 19 months from the initial analysis).
Methods: Pts with unresectable locally advanced or metastatic UC without disease progression with 4-6 cycles of 1L gemcitabine + cisplatin or carboplatin were randomized 1:1 to receive avelumab + BSC or BSC alone. The primary endpoint was OS, assessed from randomization in all pts and in pts with PD-L1+ tumors (Ventana SP263 assay). Secondary endpoints included progression-free survival (PFS) and safety.
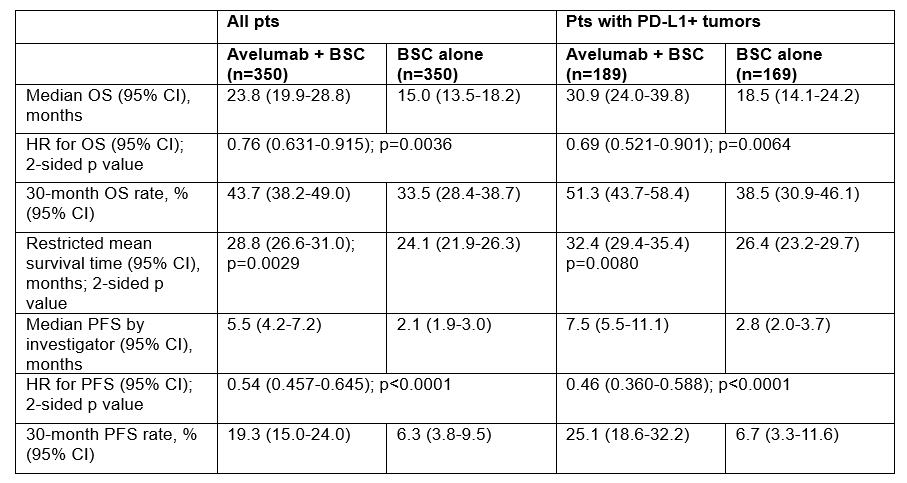
Results: 700 pts were randomized (350 per arm); 358 (51.1%) had PD-L1+ tumors. With extended follow-up (median, =38 months in both arms for all pts; data cutoff, June 4, 2021), OS remained significantly longer in the avelumab + BSC vs BSC alone arm in all randomized pts and in pts with PD-L1+ tumors (Table). An OS benefit was observed across prespecified subgroups. PFS (by investigator) was longer with avelumab + BSC vs BSC alone in all randomized pts and in pts with PD-L1+ tumors (Table). In the avelumab + BSC and BSC alone arms, respectively, 185 (52.9%) vs 252 (72.0%) pts received a subsequent anticancer drug therapy, including a PD-(L)1 inhibitor in 40 (11.4%) vs 186 (53.1%) pts. Long-term safety was consistent with previous avelumab monotherapy studies, with no new safety signals.
Conclusions: Long-term follow-up from the JAVELIN Bladder 100 trial continues to show prolonged OS with avelumab + BSC vs BSC alone. These results further support the standard-of-care role for avelumab as 1L maintenance in pts with advanced UC that has not progressed with 1L platinum-containing chemotherapy.
Source of Funding: This trial is sponsored by Pfizer as part of an alliance between Pfizer and the healthcare business of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945).
Methods: Pts with unresectable locally advanced or metastatic UC without disease progression with 4-6 cycles of 1L gemcitabine + cisplatin or carboplatin were randomized 1:1 to receive avelumab + BSC or BSC alone. The primary endpoint was OS, assessed from randomization in all pts and in pts with PD-L1+ tumors (Ventana SP263 assay). Secondary endpoints included progression-free survival (PFS) and safety.
Results: 700 pts were randomized (350 per arm); 358 (51.1%) had PD-L1+ tumors. With extended follow-up (median, =38 months in both arms for all pts; data cutoff, June 4, 2021), OS remained significantly longer in the avelumab + BSC vs BSC alone arm in all randomized pts and in pts with PD-L1+ tumors (Table). An OS benefit was observed across prespecified subgroups. PFS (by investigator) was longer with avelumab + BSC vs BSC alone in all randomized pts and in pts with PD-L1+ tumors (Table). In the avelumab + BSC and BSC alone arms, respectively, 185 (52.9%) vs 252 (72.0%) pts received a subsequent anticancer drug therapy, including a PD-(L)1 inhibitor in 40 (11.4%) vs 186 (53.1%) pts. Long-term safety was consistent with previous avelumab monotherapy studies, with no new safety signals.
Conclusions: Long-term follow-up from the JAVELIN Bladder 100 trial continues to show prolonged OS with avelumab + BSC vs BSC alone. These results further support the standard-of-care role for avelumab as 1L maintenance in pts with advanced UC that has not progressed with 1L platinum-containing chemotherapy.
Source of Funding: This trial is sponsored by Pfizer as part of an alliance between Pfizer and the healthcare business of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945).


.jpg)
.jpg)