Back
Poster, Podium & Video Sessions
Podium
PD10: Bladder Cancer: Invasive II
PD10-11: Global variation in quality of transurethral resection of bladder surgery, results from the RESECT study.
Friday, May 13, 2022
2:40 PM – 2:50 PM
Location: Room 255
Kevin Gallagher, Edinburgh, United Kingdom, Nikita Bhatt, Norfolk, United Kingdom, Keiran Clement, Glasgow, United Kingdom, Eleanor Zimmermann, Plymouth, United Kingdom, Sinan Khadhouri, Aberdeen, United Kingdom, Meghana Kulkarni, London, United Kingdom, Thineskrishna Anbarasan, Edinburgh, United Kingdom, Vinson Wai-Shun Chan, Leeds, United Kingdom, Alexander Light, London, United Kingdom, Chon Meng Lam, Aqua Asif, Leicester, United Kingdom, Fortis Gaba, Glasgow, United Kingdom, Sabrina Rossi, Cambridge, United Kingdom, Chuanyu Gao, Kent, United Kingdom, Arjun Nathan, Tim O'Brien, London, United Kingdom, Steven Maclennan, Aberdeen, United Kingdom, Matthew Nielsen, Chapel Hill, NC, Paramananthan Mariappan, Edinburgh, United Kingdom, Veeru Kasivisvanathan, RESECT STUDY Global collaborators*, London, United Kingdom
- AG
Podium Presenter(s)
Introduction: RESECT is an international multicentre observational study of transurethral resection of bladder tumour (TURBT) surgery. TURBT practice has been shown to be associated with oncological outcomes. The aim of this analysis was to measure the global achievement of 4 TURBT quality indicators (QI) and determine if there is need for improvement.
Methods: Patients included were consecutive, primary (first) tumour TURBT cases undertaken with curative intent. QIs and eligibility criteria were set a priori with consensus from an expert panel and investigators were blinded to this. These were: Detrusor muscle sampled (QI1-DM+) (eligible: tumours >5mm); Single instillation intravesical chemotherapy given within 24 hours (QI2-SIIVC) (eligible: all cases unless patient allergic or SI-IVC is not available); The completeness of resection is documented (QI3-ResDoc) (eligible: all); All of tumour number, size and location are documented (QI4-TumDoc) (eligible: all). To assess if variation was similar within the largest country in the study (UK, N=69) vs internationally, we displayed performance achievement variation, graphically, in the UK and non-UK (N=49) sites.
Results: 3193 patients undergoing TURBT from 175 sites in 40 countries were included.
The achievement of each of the TURBT quality indicators had wide variation between sites both within and between countries (Figure 1). All 4 QI’s varied from <10% achievement to 100% achievement across sites. Median (25th, 75th) achievement rate across sites with > 10 cases for each QI was: QI1-DM+: 75% (59.5-85.0); QI2-SIIVC24: 41.7% (19.0-64.6); QI3-ResDoc: 80.0% (57.5-91.9); QI4-TumDoc: 68.4% (50.0-80.6).
There was low grade pathology in 134/537 (24.9%) cases where the surgeon did not request SI-IVC because of belief it was not indicated, and in 187/468 (40.0%) cases where the surgeon did not request SI-IVC without a documented reason. These cases may have benefited from SI-IVC treatment (level 1a evidence).
Conclusions: There is significant variation in the achievement of four key TURBT QI within countries and internationally with significant room for improvement. Phase 2 of the RESECT study will randomise sites to targeted feedback or not, to investigate if it is possible to improve this performance and reduce recurrence rates.
Source of Funding: Rosetrees Trust, The Urology Foundation, Karl Storz Endocopy, Photocure, Action Bladder Cancer, British Journal of Urology International Charity.
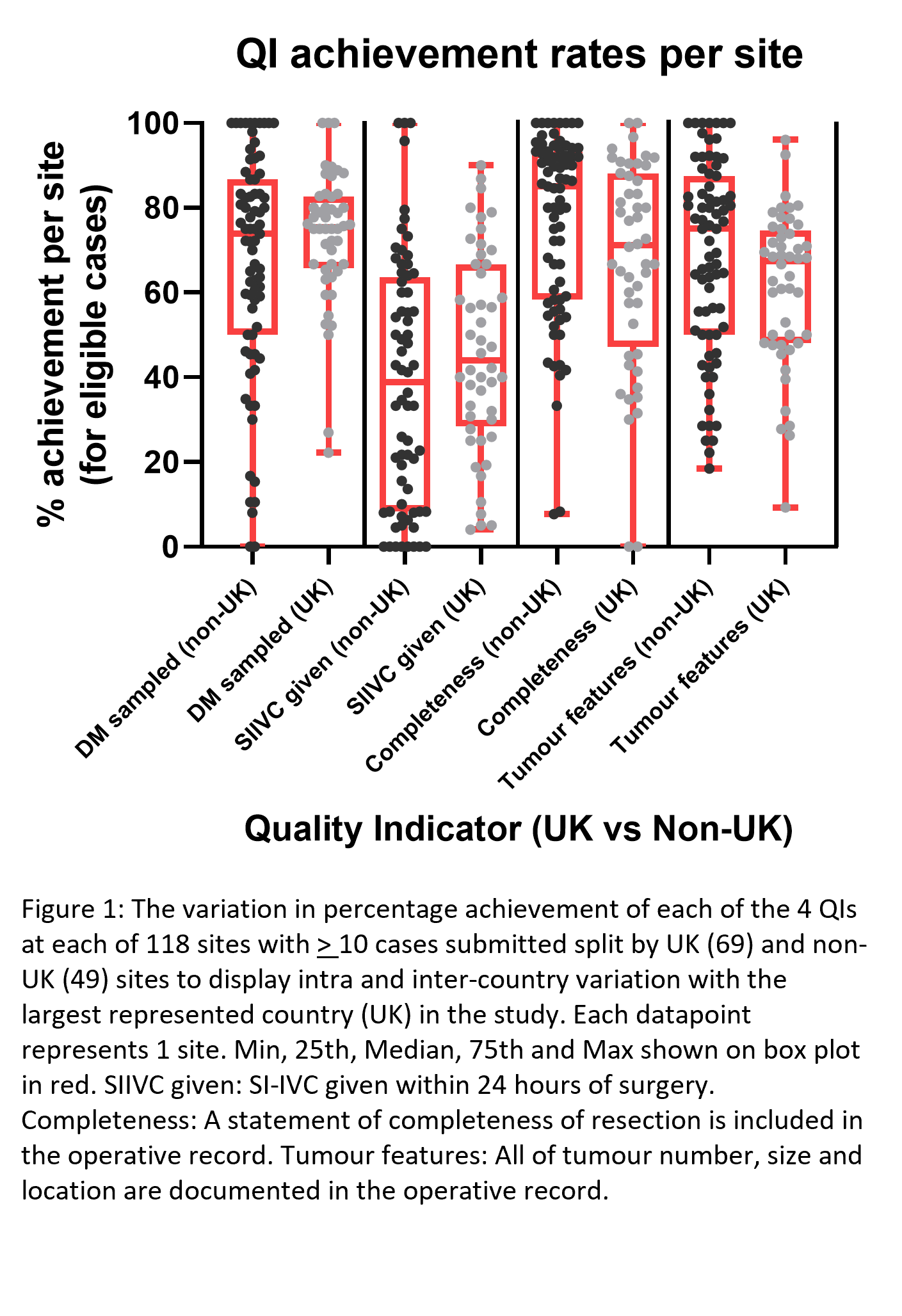
Methods: Patients included were consecutive, primary (first) tumour TURBT cases undertaken with curative intent. QIs and eligibility criteria were set a priori with consensus from an expert panel and investigators were blinded to this. These were: Detrusor muscle sampled (QI1-DM+) (eligible: tumours >5mm); Single instillation intravesical chemotherapy given within 24 hours (QI2-SIIVC) (eligible: all cases unless patient allergic or SI-IVC is not available); The completeness of resection is documented (QI3-ResDoc) (eligible: all); All of tumour number, size and location are documented (QI4-TumDoc) (eligible: all). To assess if variation was similar within the largest country in the study (UK, N=69) vs internationally, we displayed performance achievement variation, graphically, in the UK and non-UK (N=49) sites.
Results: 3193 patients undergoing TURBT from 175 sites in 40 countries were included.
The achievement of each of the TURBT quality indicators had wide variation between sites both within and between countries (Figure 1). All 4 QI’s varied from <10% achievement to 100% achievement across sites. Median (25th, 75th) achievement rate across sites with > 10 cases for each QI was: QI1-DM+: 75% (59.5-85.0); QI2-SIIVC24: 41.7% (19.0-64.6); QI3-ResDoc: 80.0% (57.5-91.9); QI4-TumDoc: 68.4% (50.0-80.6).
There was low grade pathology in 134/537 (24.9%) cases where the surgeon did not request SI-IVC because of belief it was not indicated, and in 187/468 (40.0%) cases where the surgeon did not request SI-IVC without a documented reason. These cases may have benefited from SI-IVC treatment (level 1a evidence).
Conclusions: There is significant variation in the achievement of four key TURBT QI within countries and internationally with significant room for improvement. Phase 2 of the RESECT study will randomise sites to targeted feedback or not, to investigate if it is possible to improve this performance and reduce recurrence rates.
Source of Funding: Rosetrees Trust, The Urology Foundation, Karl Storz Endocopy, Photocure, Action Bladder Cancer, British Journal of Urology International Charity.


.jpg)
.jpg)