Back
Poster, Podium & Video Sessions
Podium
PD12: Bladder Cancer: Basic Research & Pathophysiology II
PD12-10: Urine Comprehensive Genomic Profiling of urothelial carcinoma patients undergoing intravesical therapy enables detection of actionable DNA mutations associated with drug response and risk stratification
Friday, May 13, 2022
2:30 PM – 2:40 PM
Location: Room 244
Goran Rac*, Shalin Desai, Hiten D. Patel, Chirag Doshi, Ryan Dornbier, Maywood, IL, Vincent Caruso, Peter Lentz, Brian C. Mazzarella, Kevin G. Phillips, Trevor Levin, South San Francisco, CA, Alan J. Wolfe, Gopal N. Gupta, Maywood, IL

Goran Rac, MD
Loyola University Medical Center
Podium Presenter(s)
Introduction: Prediction of intravesical therapy (IVT) response and risk stratification in patients with urothelial carcinoma (UC) remains a challenge. Previous attempts using biomarkers have not gained broad adoption. Urinary comprehensive genomic profiling (uCGP) has significant potential to aid in both quantitative assessment of therapeutic response and risk stratification.
Methods: uCGP was performed on 26 prospectively enrolled patients undergoing IVT induction (BCG = 88%, Gemcitabine = 12%). Samples were collected after visually complete TURBT immediately prior to IVT instillation. Urine DNA was sequenced and profiled across 60 genes using the CLIA-validated UroAmplitude test (Convergent Genomics). Recurrence predictions were performed using a previously validated algorithm. Subjects were grouped into high, intermediate, and low risk of recurrence.
Results: Stage distribution was Tis (15%), Ta (23%), T1 (62%). Mean follow-up after IVT was 22 months. Among samples analyzed, 25% contained no detectable mutations while 75% contained =1 high confidence mutation. The high and intermediate risk classification identified 100% of pathologically confirmed recurrence. Among low recurrence risk classifications, no clinical recurrence was identified within follow-up (100% NPV). TP53 mutations were strongly correlated with post-treatment recurrence OR=28, 95%CI (1.99, 394.42), p=0.038. Two subjects had detected aneuploidy post-treatment and had T1 recurrence, supporting earlier observations of aneuploidy correlating with invasive cancer.
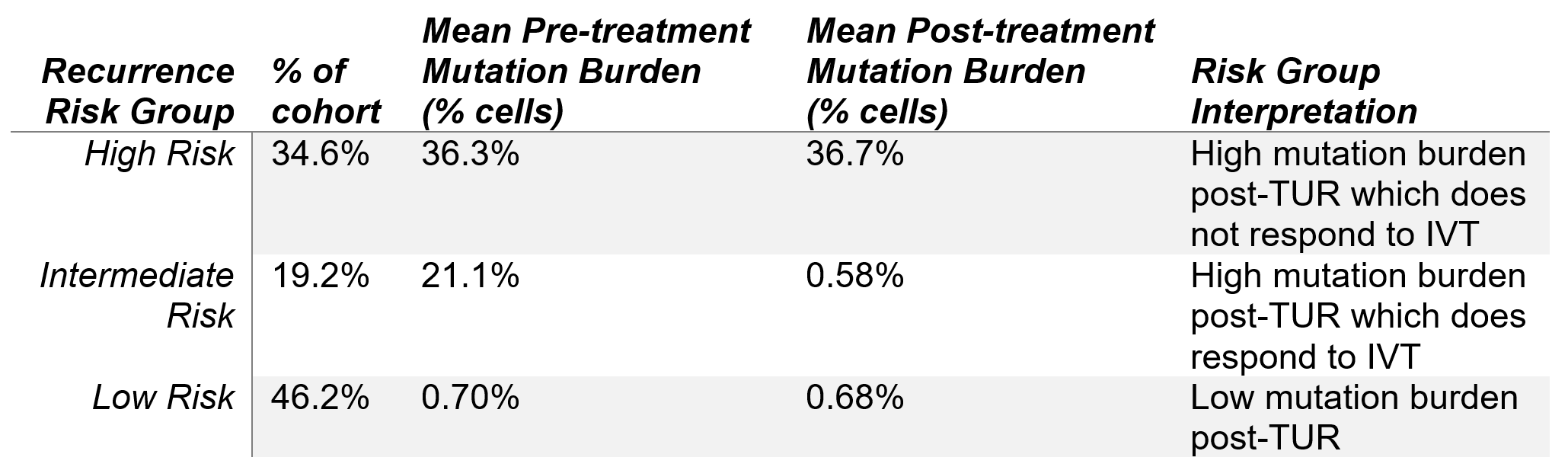
Conclusions: uCGP is a quantitative diagnostic with potential to identify therapeutic response. Low risk patients have low mutation burden prior to treatment, intermediate risk have notable disease burden pre-treatment with notable reduction after IVT, and high risk do not respond to treatment. These data suggest that uCGP performed before and after IVT has significant potential to stratify recurrence risk in non-muscle invasive UC patients. Additional studies are underway to further support the generalizability of these findings.
Source of Funding: Support for this research is provided by the NCI through a SBIR Grant, 5R44CA200174, provided to Convergent Genomics.
Methods: uCGP was performed on 26 prospectively enrolled patients undergoing IVT induction (BCG = 88%, Gemcitabine = 12%). Samples were collected after visually complete TURBT immediately prior to IVT instillation. Urine DNA was sequenced and profiled across 60 genes using the CLIA-validated UroAmplitude test (Convergent Genomics). Recurrence predictions were performed using a previously validated algorithm. Subjects were grouped into high, intermediate, and low risk of recurrence.
Results: Stage distribution was Tis (15%), Ta (23%), T1 (62%). Mean follow-up after IVT was 22 months. Among samples analyzed, 25% contained no detectable mutations while 75% contained =1 high confidence mutation. The high and intermediate risk classification identified 100% of pathologically confirmed recurrence. Among low recurrence risk classifications, no clinical recurrence was identified within follow-up (100% NPV). TP53 mutations were strongly correlated with post-treatment recurrence OR=28, 95%CI (1.99, 394.42), p=0.038. Two subjects had detected aneuploidy post-treatment and had T1 recurrence, supporting earlier observations of aneuploidy correlating with invasive cancer.
Conclusions: uCGP is a quantitative diagnostic with potential to identify therapeutic response. Low risk patients have low mutation burden prior to treatment, intermediate risk have notable disease burden pre-treatment with notable reduction after IVT, and high risk do not respond to treatment. These data suggest that uCGP performed before and after IVT has significant potential to stratify recurrence risk in non-muscle invasive UC patients. Additional studies are underway to further support the generalizability of these findings.
Source of Funding: Support for this research is provided by the NCI through a SBIR Grant, 5R44CA200174, provided to Convergent Genomics.


.jpg)
.jpg)