Back
Poster, Podium & Video Sessions
Podium
PD14: Bladder Cancer: Epidemiology & Evaluation I
PD14-05: Long-term Follow-up of Intravesical Gemcitabine and Docetaxel as Rescue Therapy for Non-Muscle Invasive Bladder Cancer
Friday, May 13, 2022
4:10 PM – 4:20 PM
Location: Room 255
Phani T. Chevuru*, Ian M. McElree, Alexander C. Martin, Jordan R. Richards, Sarah L. Mott, Paul T. Gellhaus, Kenneth G. Nepple, Ryan L. Steinberg, Michael A. O'Donnell, Vignesh T. Packiam, Iowa City, IA
- PC
Podium Presenter(s)
Introduction: While radical cystectomy remains the preferred treatment for bacillus Calmette-Guérin (BCG) unresponsive high-risk non-muscle invasive bladder cancer (NMIBC), many patients are either unwilling or unfit to undergo surgery. Previous retrospective studies have demonstrated the efficacy of intravesical gemcitabine and docetaxel (Gem/Doce) for treating NMIBC after BCG failure but only reported moderate-length follow-up. We describe the long-term outcomes of patients treated with intravesical Gem/Doce after BCG failure.
Methods: We retrospectively identified patients at our institution treated with Gem/Doce for high-risk NMIBC after BCG failure between 2009 and 2017. Patients received six weekly intravesical Gem/Doce instillations. Monthly maintenance for 2 years was initiated if disease free at first follow-up. Surveillance was performed according to American Urological Association guidelines. Outcomes included high-grade recurrence-free survival (HG-RFS), progression-free survival, cystectomy-free survival, cancer-specific survival and overall survival. Recurrence was defined as pathologically confirmed tumor relapse in the bladder or prostatic urethra. Progression was defined as recurrence of disease with stage T2 or greater, cystectomy or death due to bladder cancer. Survival probabilities were calculated with the Kaplan-Meier method, indexed from the first Gem/Doce instillation.
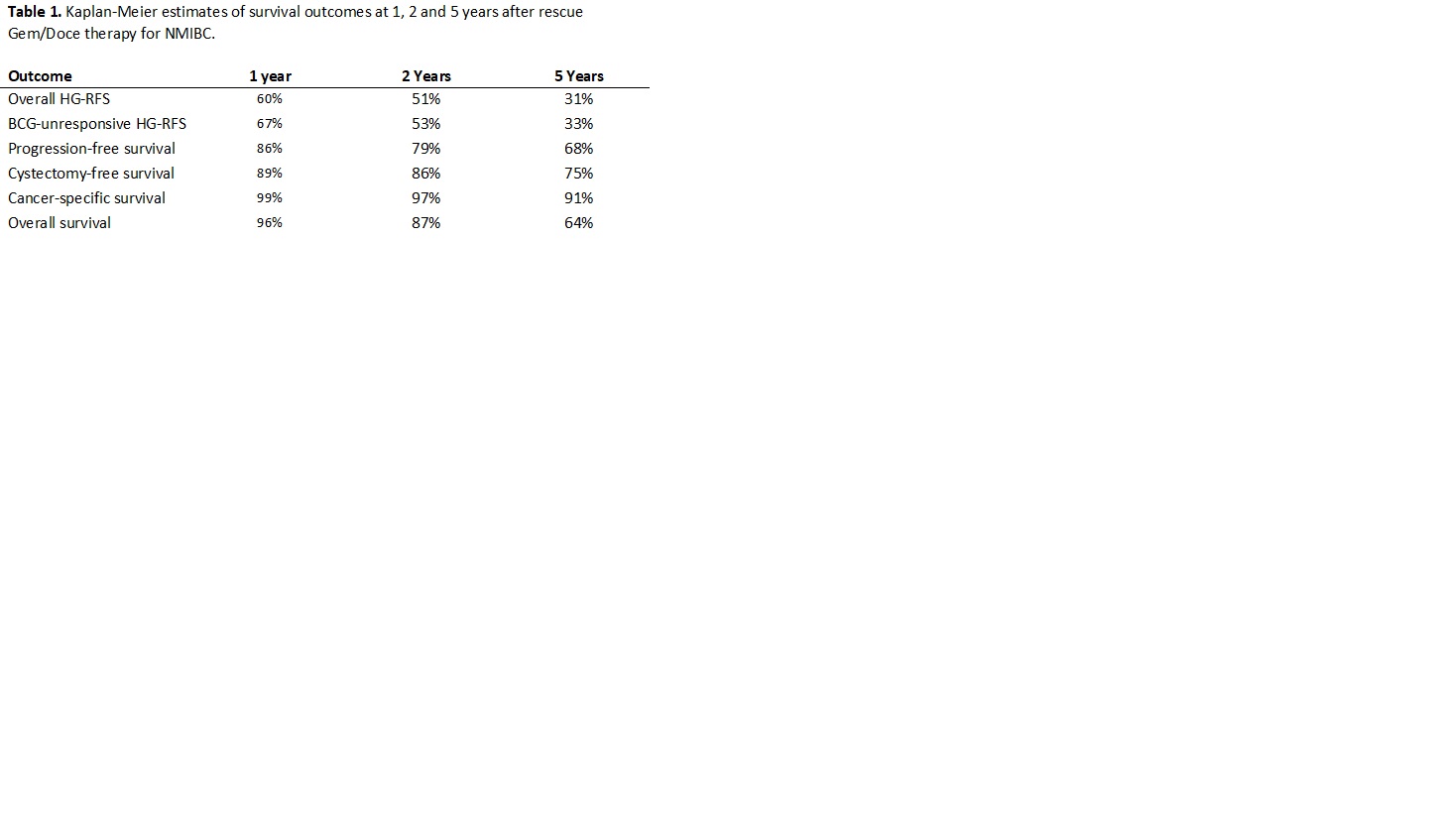
Results: A total of 97 patients with a median age of 73 years and median follow-up of 49 months (IQR: 29 – 62) were treated with Gem/Doce after BCG failure. BCG-unresponsive and BCG-relapsing disease comprised 35% and 38% of the cohort, respectively. Patients with carcinoma in-situ comprised 71% of the cohort. Complete response at initial 3-month surveillance was 74% and median duration of response was 26 months. During follow-up, 18 patients (19%) underwent radical cystectomy and 28 patients (29%) experienced disease progression. Survival outcomes are summarized in Table 1.
Conclusions: Intravesical Gem/Doce for high-risk NMIBC after BCG failure offers long-term efficacy with substantial likelihood of bladder preservation at five years after induction while maintaining excellent cancer-specific survival. Future prospective trials assessing Gem/Doce are warranted.
Source of Funding: John & Carol Walter Family Foundation and the Carver College of Medicine
Methods: We retrospectively identified patients at our institution treated with Gem/Doce for high-risk NMIBC after BCG failure between 2009 and 2017. Patients received six weekly intravesical Gem/Doce instillations. Monthly maintenance for 2 years was initiated if disease free at first follow-up. Surveillance was performed according to American Urological Association guidelines. Outcomes included high-grade recurrence-free survival (HG-RFS), progression-free survival, cystectomy-free survival, cancer-specific survival and overall survival. Recurrence was defined as pathologically confirmed tumor relapse in the bladder or prostatic urethra. Progression was defined as recurrence of disease with stage T2 or greater, cystectomy or death due to bladder cancer. Survival probabilities were calculated with the Kaplan-Meier method, indexed from the first Gem/Doce instillation.
Results: A total of 97 patients with a median age of 73 years and median follow-up of 49 months (IQR: 29 – 62) were treated with Gem/Doce after BCG failure. BCG-unresponsive and BCG-relapsing disease comprised 35% and 38% of the cohort, respectively. Patients with carcinoma in-situ comprised 71% of the cohort. Complete response at initial 3-month surveillance was 74% and median duration of response was 26 months. During follow-up, 18 patients (19%) underwent radical cystectomy and 28 patients (29%) experienced disease progression. Survival outcomes are summarized in Table 1.
Conclusions: Intravesical Gem/Doce for high-risk NMIBC after BCG failure offers long-term efficacy with substantial likelihood of bladder preservation at five years after induction while maintaining excellent cancer-specific survival. Future prospective trials assessing Gem/Doce are warranted.
Source of Funding: John & Carol Walter Family Foundation and the Carver College of Medicine


.jpg)
.jpg)