Back
Poster, Podium & Video Sessions
Podium
PD26: Bladder Cancer: Non-invasive I
PD26-03: Sequential Intravesical Gemcitabine and Docetaxel for BCG-Naïve High-Risk Non-Muscle Invasive Bladder Cancer
Saturday, May 14, 2022
1:20 PM – 1:30 PM
Location: Room 252
Ian M. McElree*, Ryan L. Steinberg, Alexander C. Martin, Jordan Richards, Sarah L. Mott, Paul T. Gellhaus, Kenneth G. Nepple, Michael A. O'Donnell, Vignesh T. Packiam, Iowa City, IA

Ian Mitchell Mcelree, BS, MS
The University of Iowa
Podium Presenter(s)
Introduction: Bacillus Calmette-Guerin (BCG) is currently recommended as adjuvant therapy following complete transurethral resection of bladder tumor (TURBT) for high-risk non-muscle invasive bladder cancer (NMIBC). However, production shortages have precluded BCG in many urologic practices. In response to the BCG shortage, Gem/Doce has been utilized at our institution in the BCG-naïve setting. We report the outcomes of patients with high-risk BCG-naïve NMIBC treated with Gem/Doce.
Methods: We retrospectively reviewed all patients with BCG-naïve high-risk NMIBC treated with Gem/Doce from May 2013 through April 2021. Patients received 6 weekly intravesical instillations of sequential 1 gram gemcitabine and 37.5 mg docetaxel after complete TURBT. Monthly maintenance of 2 years was initiated if disease free at first follow-up. The primary outcome was recurrence-free survival (RFS). Recurrence was defined as tumor relapse in the bladder or prostatic urethra. Progression was defined as T-stage increase or development of muscle invasive or metastatic disease. Survival was assessed with the Kaplan-Meier method, indexed from the first Gem/Doce instillation (p < 0.05).
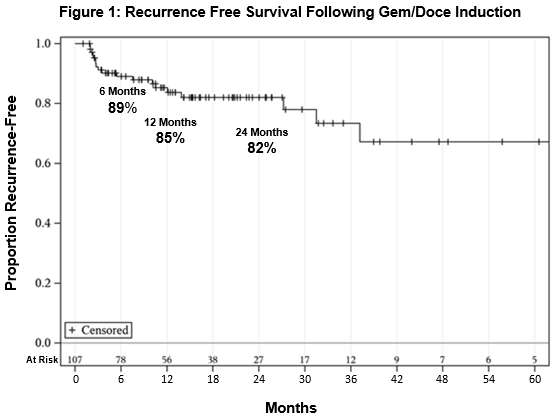
Results: One hundred seven patients with median follow-up of 15 months (IQR 8-26 months) were included in the analysis. There were 47 with any CIS, 55 with T1 disease, and 7 with micropapillary variant histology. Four patients did not complete a full induction cycle due to hematuria (3) and severe frequency/nocturia (1). RFS was 89%, 85%, and 82% at 6, 12, and 24 months, respectively (Figure 1). No difference in RFS was seen in patients with or without CIS (p=0.42). No patients had disease progression. One patient underwent cystectomy due to end-stage lower urinary tract symptoms (final pathology pTisN0). No patients died of bladder cancer. Overall survival was 84% at 24 months. 46 patients reported any symptoms during treatment, commonly urinary frequency/urgency (36%), hematuria (11%), and dysuria (8%).
Conclusions: In a large cohort of high-risk, BCG-naïve NMIBC patients, Gem/Doce showed excellent efficacy and durability (82% 2-year RFS). Prospective comparative analysis of Gem/Doce in BCG-naïve populations is warranted.
Source of Funding: This work was supported by the John & Carol Walter Family Foundation and the Carver College of Medicine.
Methods: We retrospectively reviewed all patients with BCG-naïve high-risk NMIBC treated with Gem/Doce from May 2013 through April 2021. Patients received 6 weekly intravesical instillations of sequential 1 gram gemcitabine and 37.5 mg docetaxel after complete TURBT. Monthly maintenance of 2 years was initiated if disease free at first follow-up. The primary outcome was recurrence-free survival (RFS). Recurrence was defined as tumor relapse in the bladder or prostatic urethra. Progression was defined as T-stage increase or development of muscle invasive or metastatic disease. Survival was assessed with the Kaplan-Meier method, indexed from the first Gem/Doce instillation (p < 0.05).
Results: One hundred seven patients with median follow-up of 15 months (IQR 8-26 months) were included in the analysis. There were 47 with any CIS, 55 with T1 disease, and 7 with micropapillary variant histology. Four patients did not complete a full induction cycle due to hematuria (3) and severe frequency/nocturia (1). RFS was 89%, 85%, and 82% at 6, 12, and 24 months, respectively (Figure 1). No difference in RFS was seen in patients with or without CIS (p=0.42). No patients had disease progression. One patient underwent cystectomy due to end-stage lower urinary tract symptoms (final pathology pTisN0). No patients died of bladder cancer. Overall survival was 84% at 24 months. 46 patients reported any symptoms during treatment, commonly urinary frequency/urgency (36%), hematuria (11%), and dysuria (8%).
Conclusions: In a large cohort of high-risk, BCG-naïve NMIBC patients, Gem/Doce showed excellent efficacy and durability (82% 2-year RFS). Prospective comparative analysis of Gem/Doce in BCG-naïve populations is warranted.
Source of Funding: This work was supported by the John & Carol Walter Family Foundation and the Carver College of Medicine.

.jpg)
.jpg)