Back
Poster, Podium & Video Sessions
Podium
PD29: Trauma/Reconstruction/Diversion: External Genitalia Reconstruction and Urotrauma (including transgender surgery) II
PD29-08: Penuma&[copy] implant surgery - a safety and efficacy trial
Saturday, May 14, 2022
2:10 PM – 2:20 PM
Location: Room 244
Ariel Zisman*, Alexandra Siegal, Shirin Razdan, Robert Valenzuela, New York, NY

Ariel Zisman, MD
Fellow
Rambam Health Care Campus, Haifa, Israel
Podium Presenter(s)
Introduction: Penuma® is a silicone penile implant that is FDA approved for the correction of cosmetic deformities of the penis, such as retractile penis, as well as enhancing the girth and length of flaccid penis. The increasing popularity of the device is accompanied by concerns over patient safety. Possible risks and complications include: infection or erosion requiring removal of the implant, hematoma, seroma, prosthesis displacement, and "wing"-shaped creases necessitating revision. Presented here are our initial experiences with patients undergoing Penuma® implant surgery.
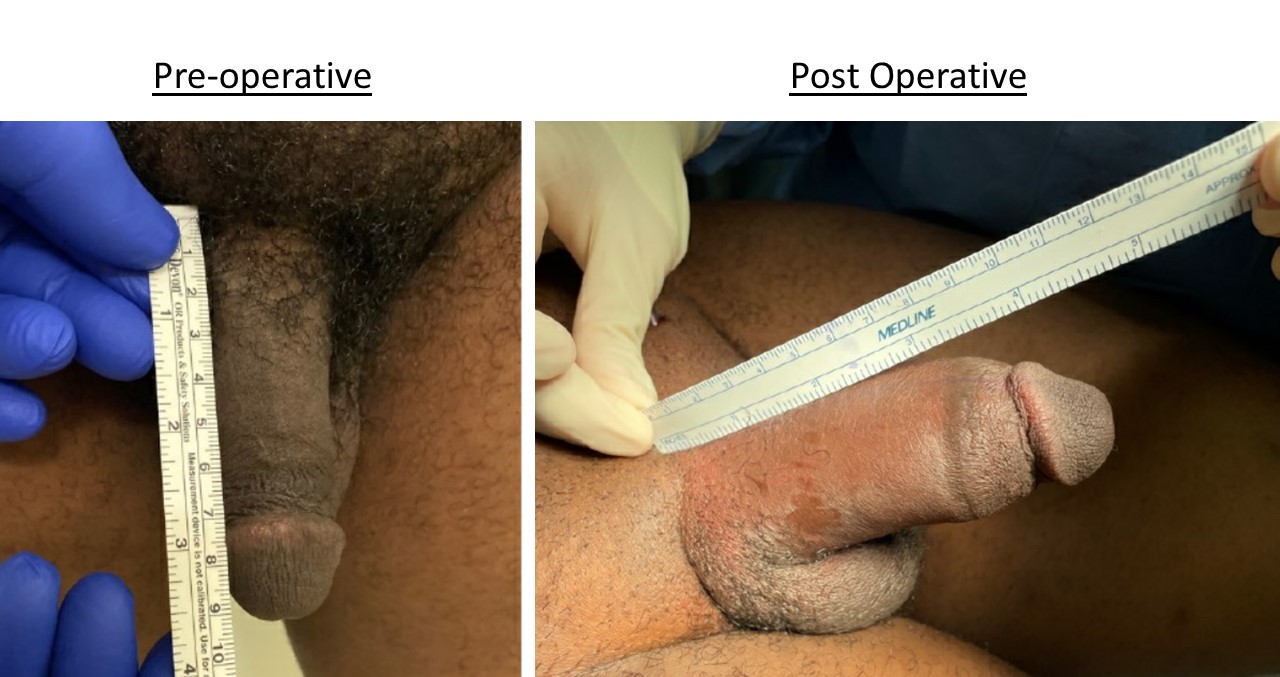
Methods: We performed a retrospective analysis of men undergoing Penuma® implants from March-September 2021. Exclusion criteria included uncircumcised patients and those who have had previous penile enhancement surgery that caused excess scarring. Uncircumcised patients were offered to undergo circumcision prior to surgery. Data was collected on demographics as well as body mass index (BMI) and comorbidities. Measurements of penile length were made both pre- and post-operatively.
Results: Between March and September 2021, 32 male patients underwent Penuma® penile implant surgery. Mean patient age was 39±9 years. Mean BMI was 28±4. All but two patients were non-smokers and only one had a comorbidity (diabetes). A scrotal approach was used in 23 cases (71%) and the rest were performed with an infrapubic approach. Post-operative measurements were available for 19 patients. Preoperative mean flaccid length was 8.3±2 cm. Postoperative mean length was 13±1.8 cm. Patients added an average of 4.8±1.6 cm to their penile length. Average follow up time was 4.4 months. Among the complications were 1 case of erosion, 1 case of prosthesis displacement, and 1 case of penile "wings". Revision surgery was performed in each case, with a good cosmetic outcome.
Conclusions: A Penuma® implant can be safely used to enhance flaccid penile length in patients with retractile penis or other cosmetic deformities via a scrotal or infrapubic approach.
Source of Funding: None
Methods: We performed a retrospective analysis of men undergoing Penuma® implants from March-September 2021. Exclusion criteria included uncircumcised patients and those who have had previous penile enhancement surgery that caused excess scarring. Uncircumcised patients were offered to undergo circumcision prior to surgery. Data was collected on demographics as well as body mass index (BMI) and comorbidities. Measurements of penile length were made both pre- and post-operatively.
Results: Between March and September 2021, 32 male patients underwent Penuma® penile implant surgery. Mean patient age was 39±9 years. Mean BMI was 28±4. All but two patients were non-smokers and only one had a comorbidity (diabetes). A scrotal approach was used in 23 cases (71%) and the rest were performed with an infrapubic approach. Post-operative measurements were available for 19 patients. Preoperative mean flaccid length was 8.3±2 cm. Postoperative mean length was 13±1.8 cm. Patients added an average of 4.8±1.6 cm to their penile length. Average follow up time was 4.4 months. Among the complications were 1 case of erosion, 1 case of prosthesis displacement, and 1 case of penile "wings". Revision surgery was performed in each case, with a good cosmetic outcome.
Conclusions: A Penuma® implant can be safely used to enhance flaccid penile length in patients with retractile penis or other cosmetic deformities via a scrotal or infrapubic approach.
Source of Funding: None


.jpg)
.jpg)