Back
Poster, Podium & Video Sessions
Podium
PD34: Urodynamics/Lower Urinary Tract Dysfunction/Female Pelvic Medicine: Female Incontinence: Therapy II
PD34-01: Sacral Neuromodulation Devices Consistently Harbor Bacteria and Possess a Unique Microbe and Metabolite Profile
Saturday, May 14, 2022
3:30 PM – 3:40 PM
Location: Room 244
Glenn T. Werneburg*, Ava Adler, Daniel Hettel, Sandip Vasavada, Raymond Rackley, Howard Goldman, Jacqueline Zillioux, Bradley Gill, Daniel Shoskes, Aaron Miller, Cleveland, OH

Glenn T. Werneburg, MD, PHD
Cleveland Clinic
Podium Presenter(s)
Introduction: Sacral neuromodulation (SNM) is an effective therapy for refractory overactive bladder. 25% of explanted SNM devices have been shown to harbor microbes, but only 2% develop infection requiring removal. To understand the transition from the colonized (wherein bacteria are present but not pathological) to infected state, we sought to determine microbial biofilm and metabolite composition on devices, and its association with clinical factors. We hypothesized SNM devices would consistently harbor bacteria, and possess unique bacterial/metabolite profiles.
Methods: Urological patients scheduled to undergo removal or revision of SNM devices were consented per IRB-approved protocol. Devices were swabbed intraoperatively, with safeguards to avoid contamination. Swab samples, alongside controls, were subjected to next-generation sequencing and metabolomics. Association between microbial diversity and clinical variables was then analyzed using t-tests and ANOVA.
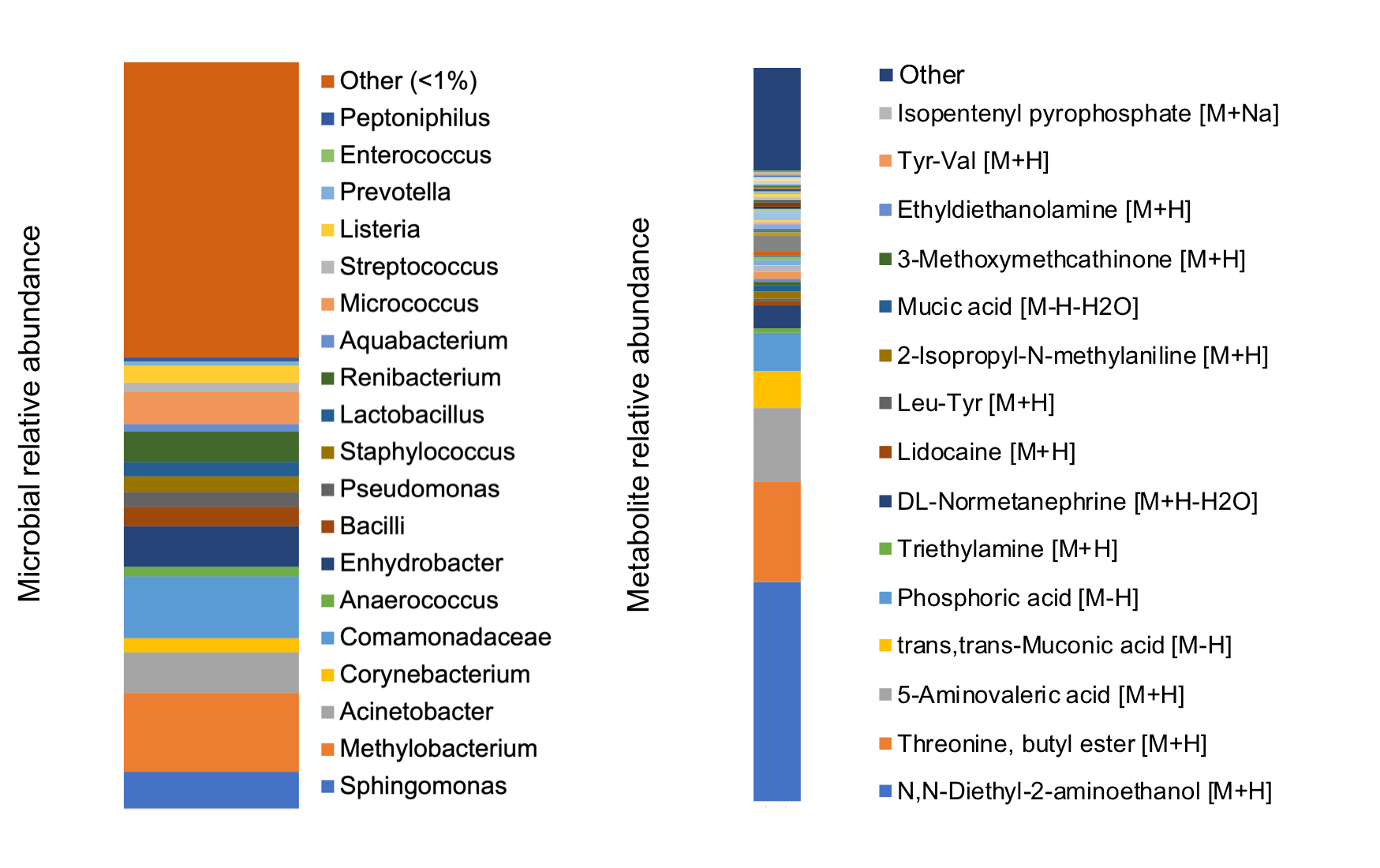
Results: 11 devices explanted for non-infectious reasons were included, and all harbored microbiota. Common genera included Acinetobacter, Pseudomonas, Corynebacterium, Staphylococcus, among others (Figure left). Diethanolamine (local anesthetic precursor), threonine-butyl-ester (peptide synthesis precursor), and 5-aminovaleric acid (bacterial metabolite of lysine; GABA agonist) were the most commonly detected metabolites (Figure right). There was no significant difference in bacterial diversity based on age, antibiotics, or BMI. SNM devices exhibited a unique microbial (p=0.047) and metabolomic (p=0.045) profile relative to other urologic device types (stents, sphincters, prostheses).
Conclusions: Explanted devices consistently harbored microbiota and possessed a unique microbe and metabolite profile. Some of the most commonly detected microbes in the non-infected devices have been previously implicated in SNM device-associated infections (Staphylococcus, Pseudomonas). Our results open new avenues for investigation of biochemical changes occurring during the transition from colonization to clinical infection, and identify preventive strategies. Future studies will compare SNM devices explanted due to infection to those removed for other reasons to understand this pathophysiology.
Source of Funding: N/A
Methods: Urological patients scheduled to undergo removal or revision of SNM devices were consented per IRB-approved protocol. Devices were swabbed intraoperatively, with safeguards to avoid contamination. Swab samples, alongside controls, were subjected to next-generation sequencing and metabolomics. Association between microbial diversity and clinical variables was then analyzed using t-tests and ANOVA.
Results: 11 devices explanted for non-infectious reasons were included, and all harbored microbiota. Common genera included Acinetobacter, Pseudomonas, Corynebacterium, Staphylococcus, among others (Figure left). Diethanolamine (local anesthetic precursor), threonine-butyl-ester (peptide synthesis precursor), and 5-aminovaleric acid (bacterial metabolite of lysine; GABA agonist) were the most commonly detected metabolites (Figure right). There was no significant difference in bacterial diversity based on age, antibiotics, or BMI. SNM devices exhibited a unique microbial (p=0.047) and metabolomic (p=0.045) profile relative to other urologic device types (stents, sphincters, prostheses).
Conclusions: Explanted devices consistently harbored microbiota and possessed a unique microbe and metabolite profile. Some of the most commonly detected microbes in the non-infected devices have been previously implicated in SNM device-associated infections (Staphylococcus, Pseudomonas). Our results open new avenues for investigation of biochemical changes occurring during the transition from colonization to clinical infection, and identify preventive strategies. Future studies will compare SNM devices explanted due to infection to those removed for other reasons to understand this pathophysiology.
Source of Funding: N/A


.jpg)
.jpg)