Back
Poster, Podium & Video Sessions
Podium
PD35: Prostate Cancer: Advanced (including Drug Therapy) II
PD35-05: Initial experience of [177Lu]Ludotadipep treatment in patients with [18F]PSMA PET CT positive metastatic castration-resistant prostate cancer: preliminary study
Sunday, May 15, 2022
7:40 AM – 7:50 AM
Location: Room 252
Dongho Shin*, Chang Eil Yoon, Hyeok Jae Kwon, Hyong Woo Moon, Yong Hyun Park, Woong Jin Bae, Hyuk Jin Cho, U-syn Ha, Sung-Hoo Hong, Sae Woong Kim, Ji Youl Lee, Seoul, Korea, Republic of
- DS
Dong Ho Shin, MD
College of Medicine, The Catholic University of Korea
Podium Presenter(s)
Introduction: Lutetium-177 labeled prostate specific membrane antigen radio ligand therapy ([177Lu]Ludotadipep), which enables targeted delivery of beta-particle radiation to prostate cancer, has been suggested as a promising novel systemic radionuclide therapy in patients with mCRPC.
Methods: From november 2020 to august 2021, 20 men with mCRPC whom disease progressed after standard treatments were recruited for screening and 18 patients were eligible for treatment. Patient underwent a Florastamin labelled prostate specific membrane antigen positron emission tomography ([18F]PSMA PET CT) for screening to confirm high PSMA-expression. Six different patients were treated with [177Lu]Ludotadipep at doses of 50mCi, 75mCi and 100mCi, respectively. [177Lu]Ludotadipep was injected via venous injection and they were hospitalized for 3days after the injection to monitoring for any adverse effects. To evaluate the treatment outcome, serum PSA levels were followed up in 1st, 2nd, 3rd, 4th, 6th, 8th, 12th weeks and [18F]PSMA PET CT was taken in 4th and 8th weeks.
Results: All patients showed [18F]PSMA PET CT positive in pre [177Lu]Ludotadipep treatment. 13 (72%) underwent radical prostatectomy. 6 (33%) had received docetaxel chemotherapy. 14 (77.8%) received prior 1st and 2nd androgen deprivation therapy. 5 (27.8%) patients underwent radiotherapy (RT). In 50mCi treated group, 40% of patients achieved PSA partial response and non-progressive disease was seen on [18F]PSMA PET CT in 60% of patients. In 75mCi treated group, 33.3% of patients achieved PSA partial response and non-progressive disease was seen on [18F]PSMA PET CT in 100% of patients. In 100mCi treated group, 33.3% of patients achieved PSA partial response and rest of the patient had non-progressive disease on [18F]PSMA PET CT. Only 2 patient complained transient nausea and there was no treatment related deaths. But one patient died shortly after with disease progression and could not finish the follow up.
Conclusions: The current study is in the early stages of [177Lu]Ludotadipep treatment, with a final target capacity of 150mCi. Therefore, we suggest that it could be a promising treatment with less toxicity for mCRPC patients who have not been responsive to conventional treatments.
Source of Funding: none
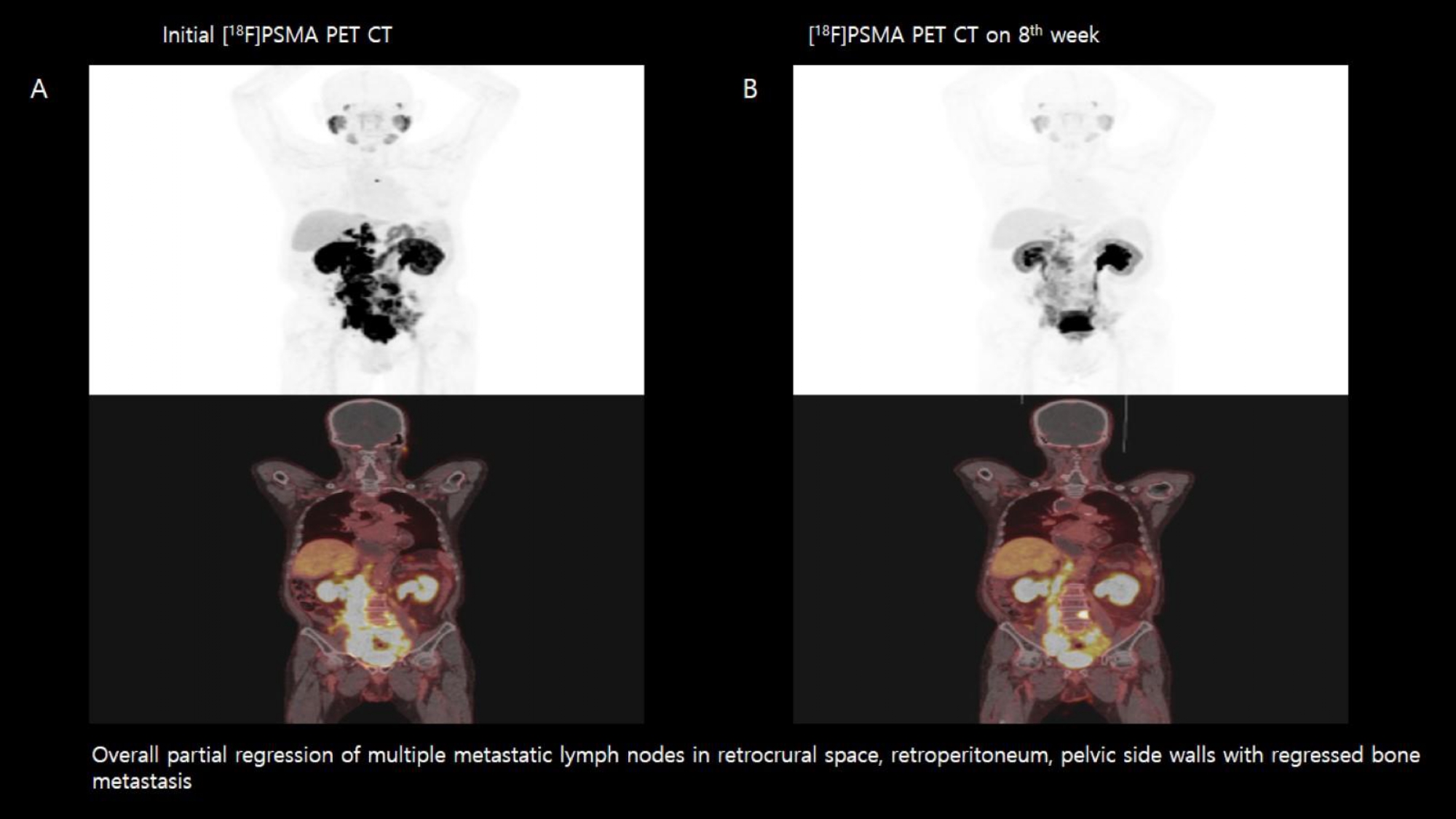
Methods: From november 2020 to august 2021, 20 men with mCRPC whom disease progressed after standard treatments were recruited for screening and 18 patients were eligible for treatment. Patient underwent a Florastamin labelled prostate specific membrane antigen positron emission tomography ([18F]PSMA PET CT) for screening to confirm high PSMA-expression. Six different patients were treated with [177Lu]Ludotadipep at doses of 50mCi, 75mCi and 100mCi, respectively. [177Lu]Ludotadipep was injected via venous injection and they were hospitalized for 3days after the injection to monitoring for any adverse effects. To evaluate the treatment outcome, serum PSA levels were followed up in 1st, 2nd, 3rd, 4th, 6th, 8th, 12th weeks and [18F]PSMA PET CT was taken in 4th and 8th weeks.
Results: All patients showed [18F]PSMA PET CT positive in pre [177Lu]Ludotadipep treatment. 13 (72%) underwent radical prostatectomy. 6 (33%) had received docetaxel chemotherapy. 14 (77.8%) received prior 1st and 2nd androgen deprivation therapy. 5 (27.8%) patients underwent radiotherapy (RT). In 50mCi treated group, 40% of patients achieved PSA partial response and non-progressive disease was seen on [18F]PSMA PET CT in 60% of patients. In 75mCi treated group, 33.3% of patients achieved PSA partial response and non-progressive disease was seen on [18F]PSMA PET CT in 100% of patients. In 100mCi treated group, 33.3% of patients achieved PSA partial response and rest of the patient had non-progressive disease on [18F]PSMA PET CT. Only 2 patient complained transient nausea and there was no treatment related deaths. But one patient died shortly after with disease progression and could not finish the follow up.
Conclusions: The current study is in the early stages of [177Lu]Ludotadipep treatment, with a final target capacity of 150mCi. Therefore, we suggest that it could be a promising treatment with less toxicity for mCRPC patients who have not been responsive to conventional treatments.
Source of Funding: none


.jpg)
.jpg)