Back
Poster, Podium & Video Sessions
Podium
PD36: Infertility: Basic Research & Pathophysiology
PD36-08: Blood Testis Barrier-Mimetic Droplet Interface Bilayers (DIBs) For Passive Permeability Prediction
Sunday, May 15, 2022
8:10 AM – 8:20 AM
Location: Room 255
Jaime Korner*, Victoria, Canada, Meghan Robinson, Vancouver, Canada, Katherine Elvira, Victoria, Canada, Ryan Flannigan, Vancouver, Canada, Stephanie Willerth, Victoria, Canada
- JK
Podium Presenter(s)
Introduction: The blood testis barrier (BTB) serves as a “gatekeeper” for the apical compartment of the seminiferous epithelium where spermatogenesis takes place. Tight junctions between adjacent Sertoli cells create the BTB, regulate transport of molecules, and protect the luminal compartment from toxins and pathogens. Investigating the impact of toxins and drugs, and the regulation of human spermatogenesis requires realistic in vitro models. The purpose of this study was to develop an artificial cellular membrane to mimic the BTB using droplet interface bilayers (DIBs) derived from human Sertoli cell lipid extracts.
Methods: Human Sertoli cells were collected from a dissociated human testicular biopsy using magnetic activated sorting for Follicle-Stimulating Hormone Receptor (FSHR). Sertoli cells were expanded in vitro and lipids were extracted using a Lipid Extraction Kit (Cell Biolabs). Permeability experiments were conducted using a poly(dimethylsiloxane) (PDMS) microfluidic device which allows for the controlled formation of pairs of differing droplet compartments connected by a phospholipid bilayer. A solution of Sertoli lipids in squalene (10 mg/mL) was used as the outer phase. Donor droplets contained fluorophore (100 µM in buffer) and acceptor droplets contained only buffer (both pH 7.4). The DIB-connected droplet pairs were formed at 50 °C and the temperature was decreased to 37 °C for observation.
Results: These results represent the first DIBs to be made using human lipid extracts and the first use of DIBs to model permeability of the BTB. We found that the BTB-mimetic DIBs were impermeable to both fluorophores tested: fluorescein and calcein. Calcein is normally membrane-impermeable and serves as a control experiment to confirm the presence of an intact phospholipid bilayer. Fluorescein has been observed to permeate DIBs made using single, synthetic lipids and intestine-mimetic DIBs but not most blood-brain barrier-mimetic DIBs.
Conclusions: DIBs can be made from human Sertoli cell lipid extracts and show great potential to more accurately model BTB permeability.
Source of Funding: Collaborative Healthy Grant
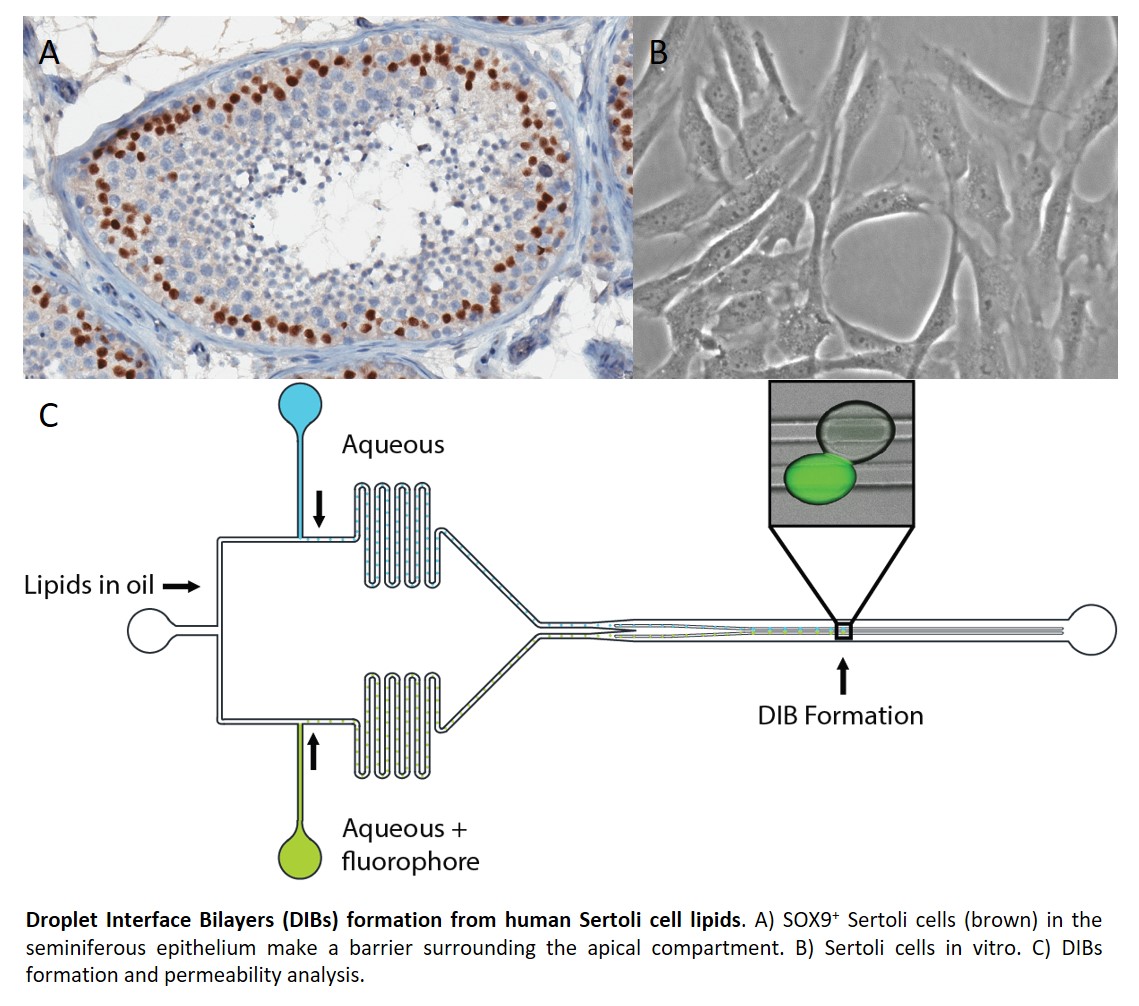
Methods: Human Sertoli cells were collected from a dissociated human testicular biopsy using magnetic activated sorting for Follicle-Stimulating Hormone Receptor (FSHR). Sertoli cells were expanded in vitro and lipids were extracted using a Lipid Extraction Kit (Cell Biolabs). Permeability experiments were conducted using a poly(dimethylsiloxane) (PDMS) microfluidic device which allows for the controlled formation of pairs of differing droplet compartments connected by a phospholipid bilayer. A solution of Sertoli lipids in squalene (10 mg/mL) was used as the outer phase. Donor droplets contained fluorophore (100 µM in buffer) and acceptor droplets contained only buffer (both pH 7.4). The DIB-connected droplet pairs were formed at 50 °C and the temperature was decreased to 37 °C for observation.
Results: These results represent the first DIBs to be made using human lipid extracts and the first use of DIBs to model permeability of the BTB. We found that the BTB-mimetic DIBs were impermeable to both fluorophores tested: fluorescein and calcein. Calcein is normally membrane-impermeable and serves as a control experiment to confirm the presence of an intact phospholipid bilayer. Fluorescein has been observed to permeate DIBs made using single, synthetic lipids and intestine-mimetic DIBs but not most blood-brain barrier-mimetic DIBs.
Conclusions: DIBs can be made from human Sertoli cell lipid extracts and show great potential to more accurately model BTB permeability.
Source of Funding: Collaborative Healthy Grant


.jpg)
.jpg)