Back
Poster, Podium & Video Sessions
Podium
PD38: Urodynamics/Lower Urinary Tract Dysfunction/Female Pelvic Medicine: Non-neurogenic Voiding Dysfunction II
PD38-11: Long-Term Efficacy and Safety of Vibegron for Overactive Bladder in Patients &[ge]65 Years Old: Analysis From the EMPOWUR Extension Trial
Sunday, May 15, 2022
8:40 AM – 8:50 AM
Location: Room 244
Jeffrey Frankel*, Burien, WA, Susann Varano, Milford, CT, Heather Greene, Elizabeth Thomas, Irvine, CA, David Staskin, Boston, MA
- JF
Jeffrey Marc Frankel, MD
Seattle Urology Research Center
Podium Presenter(s)
Introduction: Overactive bladder (OAB) prevalence increases with age. Vibegron is a ß3-adrenergic agonist approved for adults with OAB. In the phase 3 EMPOWUR extension trial, once-daily vibegron 75 mg showed long-term safety and efficacy in patients with OAB, consistent with results of the 12-week study. These post hoc analyses of the extension trial assessed safety and efficacy in patients =65 years old.
Methods: Patients completing EMPOWUR could enter the 40-week extension (NCT03583372). Patients previously receiving vibegron or tolterodine 4 mg extended release continued treatment; patients receiving placebo were randomized 1:1 to vibegron or tolterodine. The primary outcome was safety, assessed by adverse events (AEs). Key efficacy endpoints were change from EMPOWUR baseline at week 52 in average daily micturitions, urge urinary incontinence (UUI) episodes, and urgency episodes. Outcomes were assessed in patients =65 years old.
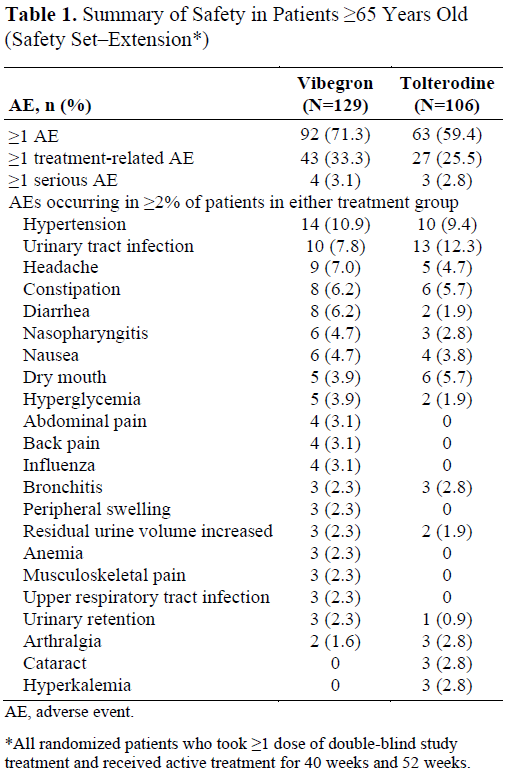
Results: Of 505 patients receiving =1 dose in the extension (n=273, vibegron; n=232, tolterodine), 235 were =65 years old. Of patients =65 years old, 71.1% were female; 75.7% had OAB wet (incontinence). After 40?52 weeks of treatment, treatment-related AE rate was similar between vibegron (33.3%) and tolterodine (25.5%) (Table 1). The most commonly occurring AEs (vibegron/tolterodine) were hypertension (10.9%/9.4%), urinary tract infection (7.8%/12.3%), and headache (7.0%/4.7%). Patients who received 52 weeks of active treatment showed improvement in least squares mean change from baseline at week 52 in micturitions (vibegron, -2.3; tolterodine, -1.7), UUI episodes (-1.8; -1.2), and urgency episodes (-3.3; -2.4) (Table 2). Safety and efficacy outcomes were generally similar in patients =75 years old.
Conclusions: In this subgroup analysis of the EMPOWUR extension trial, vibegron was associated with sustained reductions from baseline in micturitions, UUI episodes, and urgency episodes and was safe in older adults (=65 y) with OAB, consistent with the overall population.
Source of Funding: Urovant Sciences

Methods: Patients completing EMPOWUR could enter the 40-week extension (NCT03583372). Patients previously receiving vibegron or tolterodine 4 mg extended release continued treatment; patients receiving placebo were randomized 1:1 to vibegron or tolterodine. The primary outcome was safety, assessed by adverse events (AEs). Key efficacy endpoints were change from EMPOWUR baseline at week 52 in average daily micturitions, urge urinary incontinence (UUI) episodes, and urgency episodes. Outcomes were assessed in patients =65 years old.
Results: Of 505 patients receiving =1 dose in the extension (n=273, vibegron; n=232, tolterodine), 235 were =65 years old. Of patients =65 years old, 71.1% were female; 75.7% had OAB wet (incontinence). After 40?52 weeks of treatment, treatment-related AE rate was similar between vibegron (33.3%) and tolterodine (25.5%) (Table 1). The most commonly occurring AEs (vibegron/tolterodine) were hypertension (10.9%/9.4%), urinary tract infection (7.8%/12.3%), and headache (7.0%/4.7%). Patients who received 52 weeks of active treatment showed improvement in least squares mean change from baseline at week 52 in micturitions (vibegron, -2.3; tolterodine, -1.7), UUI episodes (-1.8; -1.2), and urgency episodes (-3.3; -2.4) (Table 2). Safety and efficacy outcomes were generally similar in patients =75 years old.
Conclusions: In this subgroup analysis of the EMPOWUR extension trial, vibegron was associated with sustained reductions from baseline in micturitions, UUI episodes, and urgency episodes and was safe in older adults (=65 y) with OAB, consistent with the overall population.
Source of Funding: Urovant Sciences

.jpg)
.jpg)