Back
Poster, Podium & Video Sessions
Podium
PD38: Urodynamics/Lower Urinary Tract Dysfunction/Female Pelvic Medicine: Non-neurogenic Voiding Dysfunction II
PD38-12: Long-Term Patient-Reported Outcomes of Vibegron for Overactive Bladder: Analyses From the EMPOWUR Extension Trial
Sunday, May 15, 2022
8:50 AM – 9:00 AM
Location: Room 244
David Staskin*, Boston, MA, Susann Varano, Milford, CT, Heather Greene, Elizabeth Thomas, Irvine, CA, Jeffrey Frankel, Burien, WA
Podium Presenter(s)
Introduction: Overactive bladder (OAB) may significantly impact quality of life (QoL) if not managed well. Vibegron is efficacious for OAB, shown in the 12-week phase 3 EMPOWUR trial and in the 40-week EMPOWUR extension trial. Vibegron showed significantly greater improvements vs placebo at week 12 in OAB questionnaire (OAB-q) scores and Patient Global Impression (PGI) scores, 2 patient-reported outcome (PRO) tools concerning QoL. This analysis of the EMPOWUR extension trial investigated the long-term effect of vibegron on QoL.
Methods: Patients completing EMPOWUR could enter the 40-week extension (NCT03583372) and continue vibegron 75 mg or tolterodine 4 mg extended release or be randomly assigned treatment 1:1 (if previously on placebo). The OAB-q (health-related QoL [HRQL] total [comprising coping, concern, sleep, social interaction] and symptom bother) and PGI items (Severity, Control, Frequency, Leakage, Change) were completed at baseline and at weeks 12, 24, and 52. Descriptive statistics were used to analyze change from baseline at week 52 in OAB-q HRQL total and subscale scores and PGI subscale scores among patients receiving 52 weeks of active treatment.
Results: Overall, 298 patients (n=164, vibegron; n=134, tolterodine) and 300 patients (n=166, vibegron; n=134, tolterodine) had evaluable change from baseline at week 52 OAB-q and PGI scores, respectively. Baseline PRO scores were similar between treatment groups. Improvements in OAB-q scores seen in the 12-week trial were maintained for the 40-week extension for patients continuing on vibegron for 52 weeks (Table). Improvements from baseline in the PGI subscale scores from the 12-week trial were also maintained with continuing treatment in the 40-week extension; at week 52, 69.9% of patients receiving vibegron experienced =1-category improvement in PGI-Severity; 69.9%, PGI-Control; 74.4%, PGI-Frequency; 74.8%, PGI-Leakage; and 67.6%, PGI-Change (Table).
Conclusions: Consistent with long-term improvements in OAB symptoms and a favorable safety and tolerability profile, vibegron for 52 weeks was associated with sustained and patient-perceived meaningful improvements in OAB-q and PGI scores in the EMPOWUR extension trial.
Source of Funding: Urovant Sciences
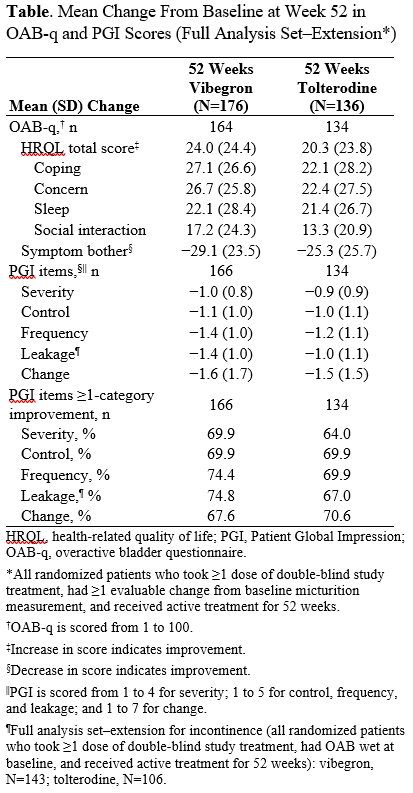
Methods: Patients completing EMPOWUR could enter the 40-week extension (NCT03583372) and continue vibegron 75 mg or tolterodine 4 mg extended release or be randomly assigned treatment 1:1 (if previously on placebo). The OAB-q (health-related QoL [HRQL] total [comprising coping, concern, sleep, social interaction] and symptom bother) and PGI items (Severity, Control, Frequency, Leakage, Change) were completed at baseline and at weeks 12, 24, and 52. Descriptive statistics were used to analyze change from baseline at week 52 in OAB-q HRQL total and subscale scores and PGI subscale scores among patients receiving 52 weeks of active treatment.
Results: Overall, 298 patients (n=164, vibegron; n=134, tolterodine) and 300 patients (n=166, vibegron; n=134, tolterodine) had evaluable change from baseline at week 52 OAB-q and PGI scores, respectively. Baseline PRO scores were similar between treatment groups. Improvements in OAB-q scores seen in the 12-week trial were maintained for the 40-week extension for patients continuing on vibegron for 52 weeks (Table). Improvements from baseline in the PGI subscale scores from the 12-week trial were also maintained with continuing treatment in the 40-week extension; at week 52, 69.9% of patients receiving vibegron experienced =1-category improvement in PGI-Severity; 69.9%, PGI-Control; 74.4%, PGI-Frequency; 74.8%, PGI-Leakage; and 67.6%, PGI-Change (Table).
Conclusions: Consistent with long-term improvements in OAB symptoms and a favorable safety and tolerability profile, vibegron for 52 weeks was associated with sustained and patient-perceived meaningful improvements in OAB-q and PGI scores in the EMPOWUR extension trial.
Source of Funding: Urovant Sciences

.jpg)
.jpg)
