Back
Poster, Podium & Video Sessions
Podium
PD42: Bladder Cancer: Invasive IV
PD42-09: Do Prophylactic Antibiotics Reduce Urinary Tract Infections Following Robot-Assisted Radical Cystectomy? A Randomized Control Trial
Sunday, May 15, 2022
10:50 AM – 11:00 AM
Location: Room 245
Holly Houenstein, Yousuf Ramahi*, Ayat A. Shah, Philippa Doherty, Kristopher Attwood, Zhe Jing, Qiang Li, Ahmed A. Hussein, Khurshid A. Guru, Buffalo, NY

Yousuf Omar Ramahi, BS
Roswell Park Comprehensive Cancer Center
Podium Presenter(s)
Introduction: Infectious complications are the most common following radical cystectomy, mostly occurring within 30 days of surgery. We sought to investigate the utility of prophylactic antibiotics to reduce urinary tract infections in the early postoperative period after Robot-Assisted Radical Cystectomy (RARC).
Methods: A prospective randomized controlled trial (RCT) was performed to investigate the safety and efficacy of prophylactic antibiotics in reducing urinary tract infections (UTI) after RARC (NCT04502095). UTI was defined as the presence of a positive urine culture (=105 cfu/mL with no more than 2 organisms) and =1 documented urinary symptom(s). Patients were randomized in a ration 1:1 to receive prophylactic antibiotics (800/160mg trimethoprim-sulfamethoxazole or 100 mg Nitrofurantonin) for 30 days, versus current standard of care (no postoperative antibiotics). Compliance of treatment group was defined as administration of antibiotic for =15 days. Data were analyzed for adverse events and development of UTI within for 30 and 90 days following RARC.
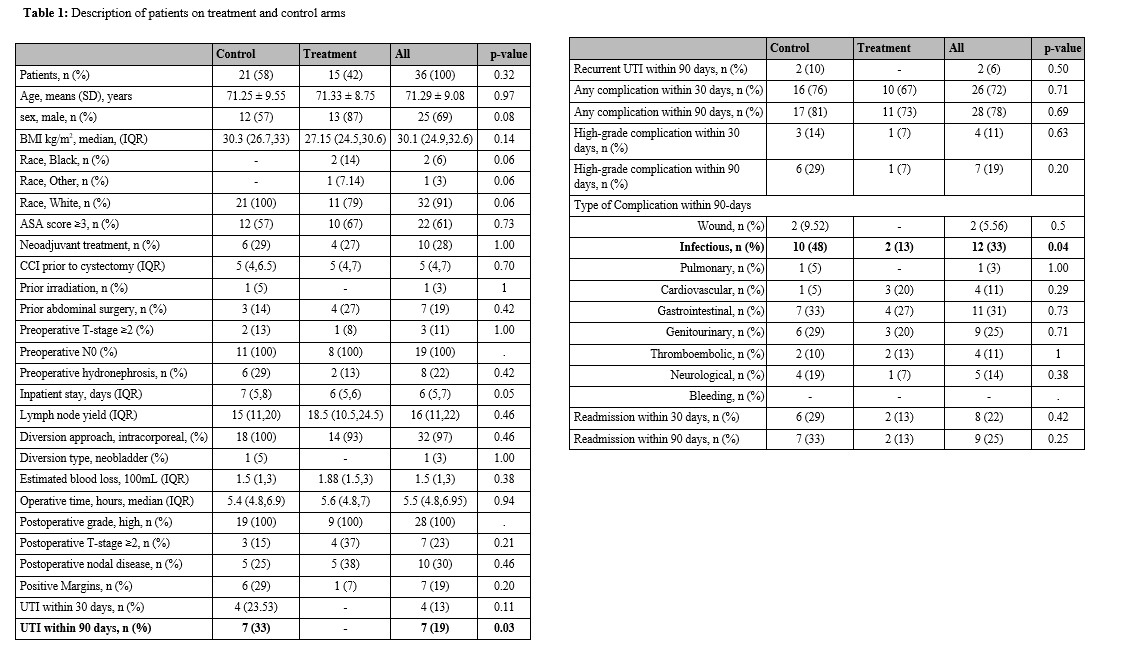
Results: 43 patients were enrolled (22 in the treatment arm and 21 controls). Final cohort comprised 36 patients (15 in treatment arm and 21 controls) with a median follow up time of 3 months (IQR 1.5-7.3). No patient in the treatment group developed UTIs within 90 days of RARC compared to 7 patients (33%) in the control group (p=0.03). Patients in the treatment group had lower 30-day (67% vs 76%, p=0.71), 90-day complications (73% vs 81%, p=0.70), 30-day (7% vs 14%, p=0.63), 90-day high-grade complications (7% vs 29%, p=0.20) and 30-day (13% vs 29%, p=0.42) and 90-day readmissions (13% vs 33%, p=0.25), but did not reach statistical significance. Patients in the treatment group experienced lower overall infectious complications within 90 days of RARC (13% vs 48%, p=0.04) (Table 1). Two (13%) patients in the treatment group experienced adverse events, one patient with Clavien-Dindo grade 2 event (Clostridium Difficile colitis), and one patient with both grade 3 (AKI) and grade 4 events (hyperkalemia). Patients with adverse events received trimethoprim-sulfamethoxazole.
Conclusions: The administration of 30-day prophylactic antibiotics after RARC was significantly associated with decreased 90-day UTIs and infectious complications after RARC.
Source of Funding: The Roswell Park Alliance Foundation
Methods: A prospective randomized controlled trial (RCT) was performed to investigate the safety and efficacy of prophylactic antibiotics in reducing urinary tract infections (UTI) after RARC (NCT04502095). UTI was defined as the presence of a positive urine culture (=105 cfu/mL with no more than 2 organisms) and =1 documented urinary symptom(s). Patients were randomized in a ration 1:1 to receive prophylactic antibiotics (800/160mg trimethoprim-sulfamethoxazole or 100 mg Nitrofurantonin) for 30 days, versus current standard of care (no postoperative antibiotics). Compliance of treatment group was defined as administration of antibiotic for =15 days. Data were analyzed for adverse events and development of UTI within for 30 and 90 days following RARC.
Results: 43 patients were enrolled (22 in the treatment arm and 21 controls). Final cohort comprised 36 patients (15 in treatment arm and 21 controls) with a median follow up time of 3 months (IQR 1.5-7.3). No patient in the treatment group developed UTIs within 90 days of RARC compared to 7 patients (33%) in the control group (p=0.03). Patients in the treatment group had lower 30-day (67% vs 76%, p=0.71), 90-day complications (73% vs 81%, p=0.70), 30-day (7% vs 14%, p=0.63), 90-day high-grade complications (7% vs 29%, p=0.20) and 30-day (13% vs 29%, p=0.42) and 90-day readmissions (13% vs 33%, p=0.25), but did not reach statistical significance. Patients in the treatment group experienced lower overall infectious complications within 90 days of RARC (13% vs 48%, p=0.04) (Table 1). Two (13%) patients in the treatment group experienced adverse events, one patient with Clavien-Dindo grade 2 event (Clostridium Difficile colitis), and one patient with both grade 3 (AKI) and grade 4 events (hyperkalemia). Patients with adverse events received trimethoprim-sulfamethoxazole.
Conclusions: The administration of 30-day prophylactic antibiotics after RARC was significantly associated with decreased 90-day UTIs and infectious complications after RARC.
Source of Funding: The Roswell Park Alliance Foundation


.jpg)
.jpg)