Back
Poster, Podium & Video Sessions
Podium
PD43: Kidney Cancer: Basic Research & Pathophysiology II
PD43-06: EZH2 inhibition as therapeutic strategy to dysregulate LATS1 in RCC
Sunday, May 15, 2022
10:20 AM – 10:30 AM
Location: Room 244
Seong Hwi Hong*, Hyun Ji Hwang, Na Gyeong Ha, Sung Yul Park, Hong Sang Moon, Young Eun Yoon, Seoul, Korea, Republic of
- SH
Podium Presenter(s)
Introduction: EZH2 is a methyl-transferase that catalyzes tri-methylation of H3K27 (histone 3 lysine 27) involved in epigenetic gene silencing. It is known to induce cancer by inhibiting the transcription of tumor suppressors. EZH2 is overexpressed in various cancers, and tazemetostat, a selective inhibitor of EZH2, is undergoing clinical trials in various urinary cancers such as bladder cancer and prostate cancer. However, it is important to clarify the effect and mechanism of tazemetostat in RCC.
Methods: We analyzed the TCGA databases of ccRCC, pRCC, and chRCC to find genetic targets of all subtype RCCs. In order to investigate the effect of tazemetostat on RCC, we checked cell viability, migration, invasion, and apoptosis using ccRCC cell lines (Caki1, 786-O, A498) and pRCC cell lines (Caki2, ACHN). Also, tissue of RCC patients was fixed by 4% paraformaldehyde for Immunohistochemistry.
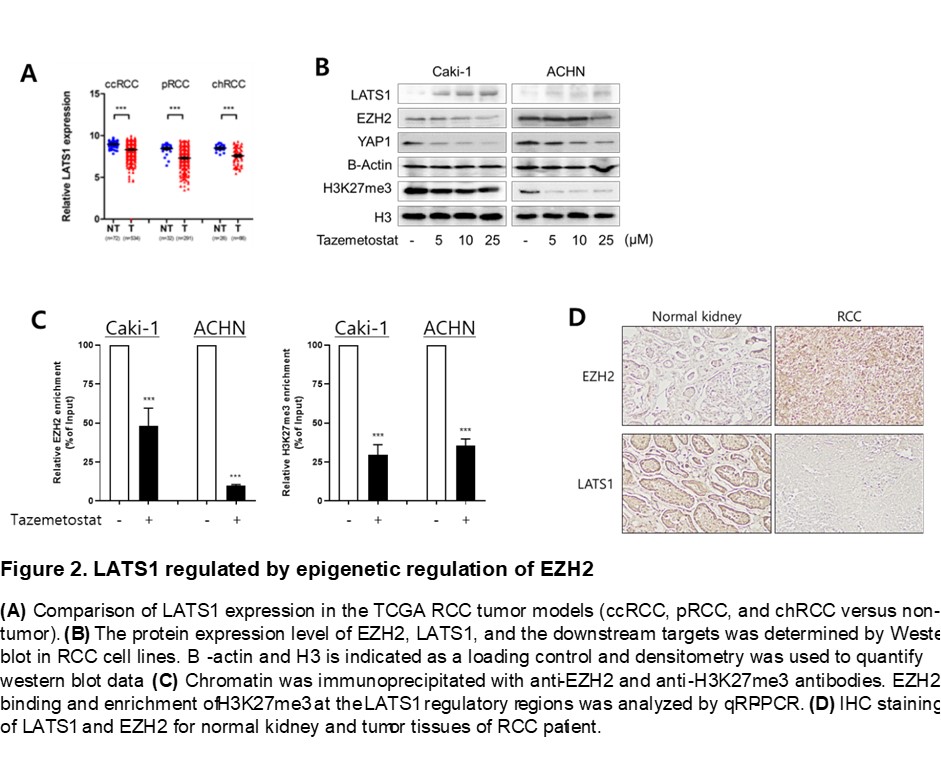
Results: In the analysis of the TCGA database, we discovered EZH2, as a key oncogene for RCCs. For RCC cell lines, tazemetostat induced the anti-tumorigenic effect. Through additional TCGA data analysis, We hypothesized LATS1, a key component of hippo pathway, as the target of EZH2. Western blot data confirmed our hypothesis. In ChIP assay, binding of EZH2 and enrichment of H3K27me3 was decreased by taxemetostat on LATS1 promoter. We showed that the same expression pattern of EZH2 and LATS1 in patient tissues.
Conclusions: In this study, EZH2 was discovered as an oncogene targeting three RCCs, and it was shown that Tazemetosat, a selective inhibitor of EZH2, epigenetically inhibits the transcription of LATS1, a tumor suppressor. The negative correlation between EZH2 and LATS1 was confirmed not only in vitro but also through tissues of RCC patients. Therefore, we expect Tazemetostat to mark a step in RCC therapy.
Source of Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education(2020R1I1A1A01067390)
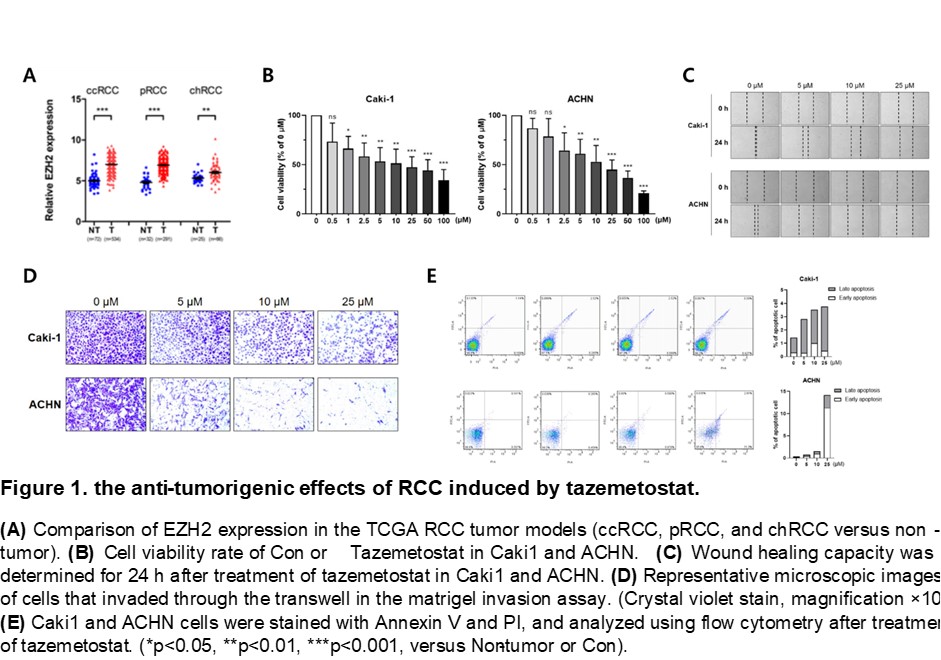
Methods: We analyzed the TCGA databases of ccRCC, pRCC, and chRCC to find genetic targets of all subtype RCCs. In order to investigate the effect of tazemetostat on RCC, we checked cell viability, migration, invasion, and apoptosis using ccRCC cell lines (Caki1, 786-O, A498) and pRCC cell lines (Caki2, ACHN). Also, tissue of RCC patients was fixed by 4% paraformaldehyde for Immunohistochemistry.
Results: In the analysis of the TCGA database, we discovered EZH2, as a key oncogene for RCCs. For RCC cell lines, tazemetostat induced the anti-tumorigenic effect. Through additional TCGA data analysis, We hypothesized LATS1, a key component of hippo pathway, as the target of EZH2. Western blot data confirmed our hypothesis. In ChIP assay, binding of EZH2 and enrichment of H3K27me3 was decreased by taxemetostat on LATS1 promoter. We showed that the same expression pattern of EZH2 and LATS1 in patient tissues.
Conclusions: In this study, EZH2 was discovered as an oncogene targeting three RCCs, and it was shown that Tazemetosat, a selective inhibitor of EZH2, epigenetically inhibits the transcription of LATS1, a tumor suppressor. The negative correlation between EZH2 and LATS1 was confirmed not only in vitro but also through tissues of RCC patients. Therefore, we expect Tazemetostat to mark a step in RCC therapy.
Source of Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education(2020R1I1A1A01067390)



.jpg)
.jpg)