Back
Poster, Podium & Video Sessions
Podium
PD43: Kidney Cancer: Basic Research & Pathophysiology II
PD43-09: Phenotypical Screening onMetastatic PRCC-TFE3 FusionTranslocation Renal Cell Carcinoma Organoids RevealsPotential Therapeutic Agents
Sunday, May 15, 2022
10:50 AM – 11:00 AM
Location: Room 244
Chuanzhen Cao*, Xiaomei Lan, Weixing Jiang, Lei Guo, Xingang Bi, Shan Zheng, Aiping Zhou, Zhijian Sun, Jianzhong Shou, Beijing, China, People's Republic of
- CC
Podium Presenter(s)
Introduction: Translocation renal cell carcinoma (tRCC) is a subtypethat occurred predominantly in children and young. Metastatic tRCC occurred in young is more aggressive and there are still no effective therapies. Organoidscan mimic original tissues then adopt to high-throughput screening (HTS). We aim to utilize patient-derived organoid and HTS to screen repurposing drugs for metastatic PRCC-TFE3 fusion tRCC.
Methods: Tumor tissues were obtained from treatment-naïve metastatic tRCC patients who underwent surgeries.Pathology, fluorescence in situ hybridization (FISH) con?rmed the PRCC-TFE3 fusion tRCC. Organoids were cultured and verified byFISH and RNA-seq.HTS was performed to seek promising drugs and potential mechanisms were analyzed by RNA-seq.
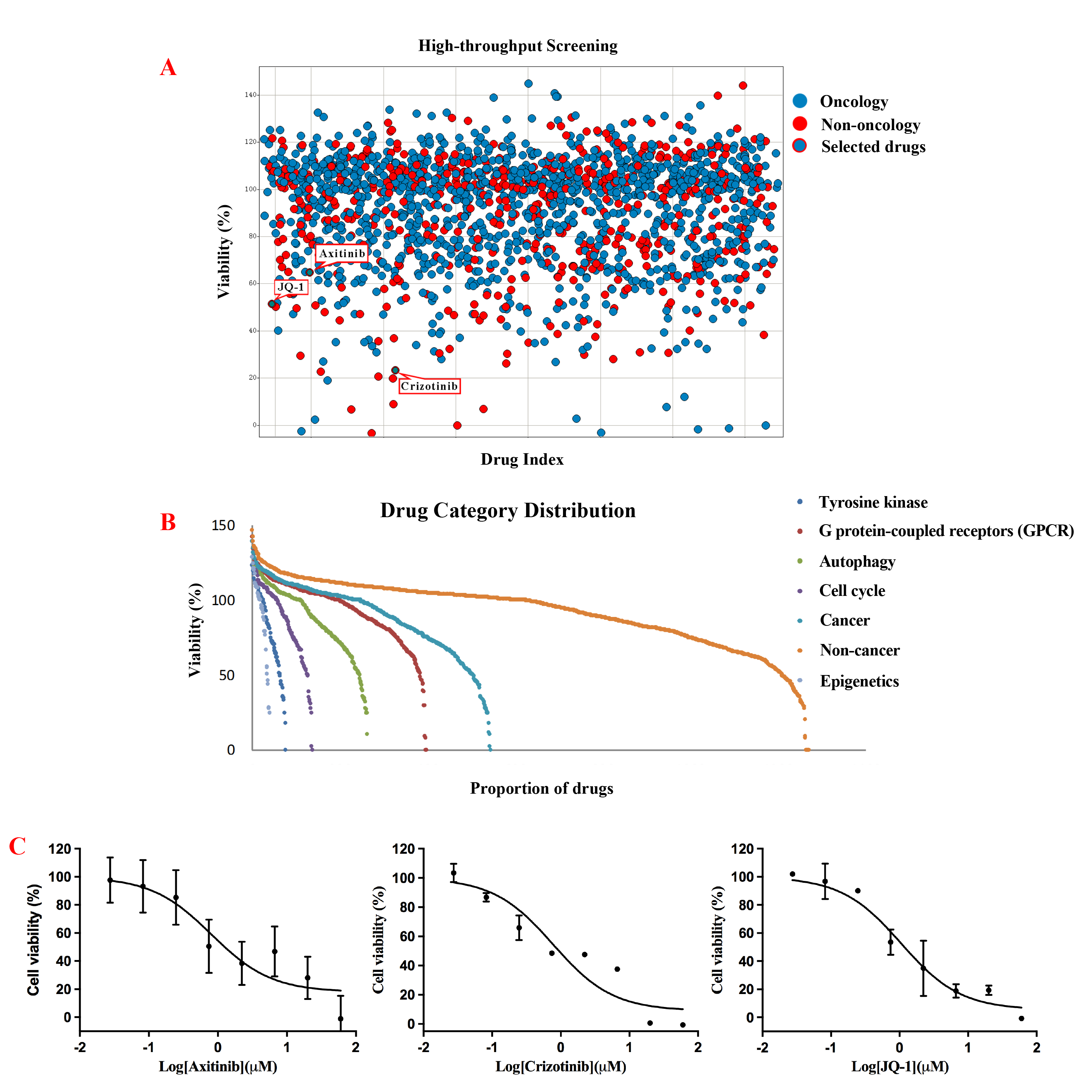
Results: We successfully established a metastatic PRCC-TFE3 fusion tRCC organoid of a common fusion subtypeand characterization was verified by histopathology, FISH,and RNA-seq. HTS assay was developed and the robustness was confirmed. A compound library of 1816 drugs was screened. Eventually, axitinib, crizotinib, and JQ-1were selected for further validation, which could induce cell cycle arrest and apoptosis. RNA-seq analyses of post-treatment organoids indicated crizotinib induced significant autophagy-related gene alternations which were consistent with tRCC potential pathogenesis.
Conclusions: We established and validated the organoid cultures from tissues starting from a metastatic PRCC-TFE3 fusion tRCC patient and achieved the HTS process for the first time. Crizotinib might be the targeted therapy worthy of exploring in clinic for metastatic PRCC-TFE3 fusion tRCC.Such organoid and HTS assay may represent a promising model system in translational research assisting clinical strategies.
Source of Funding: This work was supported by theNational Science and Technology Major Project of China (ID Number: 2016ZX09101094) and the National Natural Science Foundation of China (ID Number: 82072837).
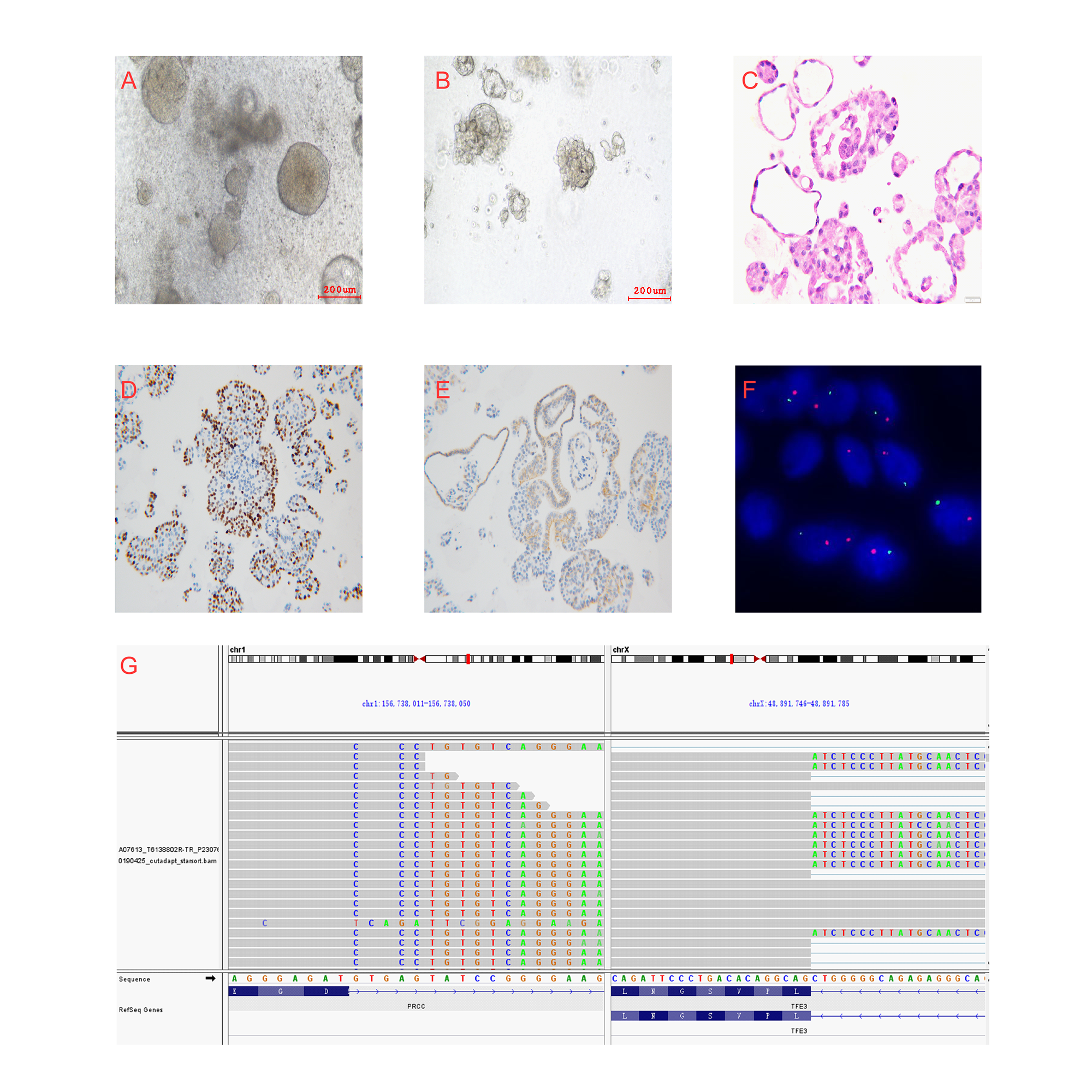
Methods: Tumor tissues were obtained from treatment-naïve metastatic tRCC patients who underwent surgeries.Pathology, fluorescence in situ hybridization (FISH) con?rmed the PRCC-TFE3 fusion tRCC. Organoids were cultured and verified byFISH and RNA-seq.HTS was performed to seek promising drugs and potential mechanisms were analyzed by RNA-seq.
Results: We successfully established a metastatic PRCC-TFE3 fusion tRCC organoid of a common fusion subtypeand characterization was verified by histopathology, FISH,and RNA-seq. HTS assay was developed and the robustness was confirmed. A compound library of 1816 drugs was screened. Eventually, axitinib, crizotinib, and JQ-1were selected for further validation, which could induce cell cycle arrest and apoptosis. RNA-seq analyses of post-treatment organoids indicated crizotinib induced significant autophagy-related gene alternations which were consistent with tRCC potential pathogenesis.
Conclusions: We established and validated the organoid cultures from tissues starting from a metastatic PRCC-TFE3 fusion tRCC patient and achieved the HTS process for the first time. Crizotinib might be the targeted therapy worthy of exploring in clinic for metastatic PRCC-TFE3 fusion tRCC.Such organoid and HTS assay may represent a promising model system in translational research assisting clinical strategies.
Source of Funding: This work was supported by theNational Science and Technology Major Project of China (ID Number: 2016ZX09101094) and the National Natural Science Foundation of China (ID Number: 82072837).




.jpg)
.jpg)