Back
Poster, Podium & Video Sessions
Podium
PD44: Urodynamics/Lower Urinary Tract Dysfunction/Female Pelvic Medicine: Pelvic Prolapse
PD44-12: Vaginal Estrogen Therapy for Genitourinary Syndrome of Menopause (GSM) in Women with a Personal History of Breast Cancer - A Claims Database Analysis
Sunday, May 15, 2022
11:20 AM – 11:30 AM
Location: Room 243
Kathryn Dumas, Sajya Singh, Jaden R Kohn, Taylor P Kohn*, Marisa Clifton, Baltimore, MD
- TK
Taylor Kohn, MD
MD, MPhil
Podium Presenter(s)
Introduction: Systemic therapies for breast cancer - selective estrogen receptor modulators (SERM), aromatase inhibitors (AI), and chemotherapies - can result in genitourinary syndrome of menopause (previously recognized as vulvovaginal atrophy) for up to 70% of postmenopausal breast cancer survivors. While systemic estrogen therapy is often avoided in these women, vaginal estrogen can be safely used to treat GSM symptoms. Our objective is to assess the use of vaginal estrogen for women with a diagnosis of GSM and a personal history of breast cancer using a large US claims database.
Methods: The TriNetX Diamond network database was queried: a US health research network of 190 million patients, encompassing healthcare encounters and prescriptions between 2009-2021. Females with a diagnosis of postmenopausal atrophic vaginitis or atrophy of the vulva (ICD-10 N95.2, N90.5) were included. Of these, women with breast cancer (C50 or Z86.000) were included if the diagnosis was at least 1 month prior to VVA diagnosis. Estrogen receptor status was collected when available (ICD-10 Z17.0, Z17.1). Prescriptions for treatment of GSM were included if prescribed the day of VVA diagnosis up to 1 year after index diagnosis. Incidence of vaginal estrogen use in patients with a history of breast cancer was determined for 2013-2015, 2016-2018, and 2019 to present.
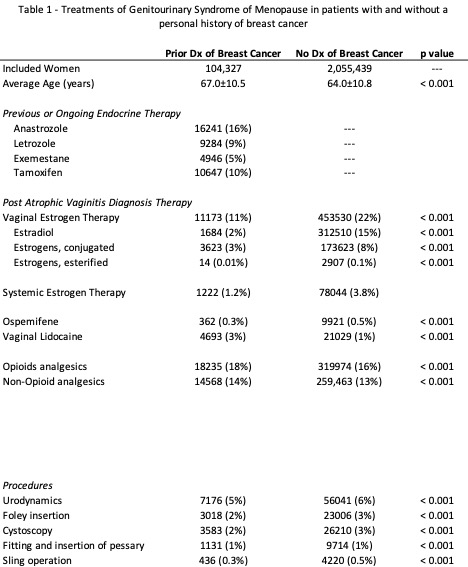
Results: A total of 2,159,766 women with GSM were identified; of whom, 4.8% (n=104,327) had a personal history of breast cancer. Vaginal estrogen prescriptions were less frequent in women with history of breast cancer compared to women without (11% vs 22%, p<0.01, Table 1). Incidence rate of vaginal estrogen prescriptions for women with GSM and history of breast cancer remained stable around 10-11% from 2013 to present. 25,410 women with GSM and history of breast cancer had positive estrogen receptor status (ER+) and 4,893 had negative estrogen receptor status (ER-). There was no difference in vaginal estrogen prescribing between ER+ and ER- patients (7.4% vs 8.4%).
Conclusions: While vaginal estrogen can be safely used in women with GSM and personal history of breast cancer, it is prescribed for few eligible patients. Barriers to use of vaginal estrogen in this population should be explored to optimize patient’s quality of life.
Source of Funding: None
Methods: The TriNetX Diamond network database was queried: a US health research network of 190 million patients, encompassing healthcare encounters and prescriptions between 2009-2021. Females with a diagnosis of postmenopausal atrophic vaginitis or atrophy of the vulva (ICD-10 N95.2, N90.5) were included. Of these, women with breast cancer (C50 or Z86.000) were included if the diagnosis was at least 1 month prior to VVA diagnosis. Estrogen receptor status was collected when available (ICD-10 Z17.0, Z17.1). Prescriptions for treatment of GSM were included if prescribed the day of VVA diagnosis up to 1 year after index diagnosis. Incidence of vaginal estrogen use in patients with a history of breast cancer was determined for 2013-2015, 2016-2018, and 2019 to present.
Results: A total of 2,159,766 women with GSM were identified; of whom, 4.8% (n=104,327) had a personal history of breast cancer. Vaginal estrogen prescriptions were less frequent in women with history of breast cancer compared to women without (11% vs 22%, p<0.01, Table 1). Incidence rate of vaginal estrogen prescriptions for women with GSM and history of breast cancer remained stable around 10-11% from 2013 to present. 25,410 women with GSM and history of breast cancer had positive estrogen receptor status (ER+) and 4,893 had negative estrogen receptor status (ER-). There was no difference in vaginal estrogen prescribing between ER+ and ER- patients (7.4% vs 8.4%).
Conclusions: While vaginal estrogen can be safely used in women with GSM and personal history of breast cancer, it is prescribed for few eligible patients. Barriers to use of vaginal estrogen in this population should be explored to optimize patient’s quality of life.
Source of Funding: None


.jpg)
.jpg)