Back
Poster, Podium & Video Sessions
Podium
PD46: Prostate Cancer: Basic Research & Pathophysiology II
PD46-09: Lipopolysaccharide from dysbiotic gut microbiota promotes inflammatory prostate cancer growth through histamine H1 receptor signaling
Sunday, May 15, 2022
2:20 PM – 2:30 PM
Location: Room 255
Makoto Matsushita*, Suita, Japan, Kazutoshi Fujita, Osakasayama, Japan, Takuji Hayashi, Hisako Kayama, Daisuke Motooka, Hiroaki Hase, Toshihiko Uemura, Akinaru Yamamoto, Gaku Yamamichi, Eisuke Tomiyama, Yoko Koh, Taigo Kato, Koji Hatano, Atsunari Kawashima, Motohide Uemura, Satoshi Nojima, Ryoichi Imamura, Kazutake Tsujikawa, Shota Nakamura, Kiyoshi Takeda, Eiichi Morii, Norio Nonomura, Suita, Japan
- MM
Podium Presenter(s)
Introduction: High-fat diet (HFD) induce prostate inflammation and promote cancer growth. But the mechanism has not been entirely clear. We have reported that diet-influenced gut microbiota regulates prostate cancer (PCa) growth via IGF-1 signaling. In this study, we investigated the cause of HFD-induced inflammatory PCa growth and the role of gut microbiota in this process.
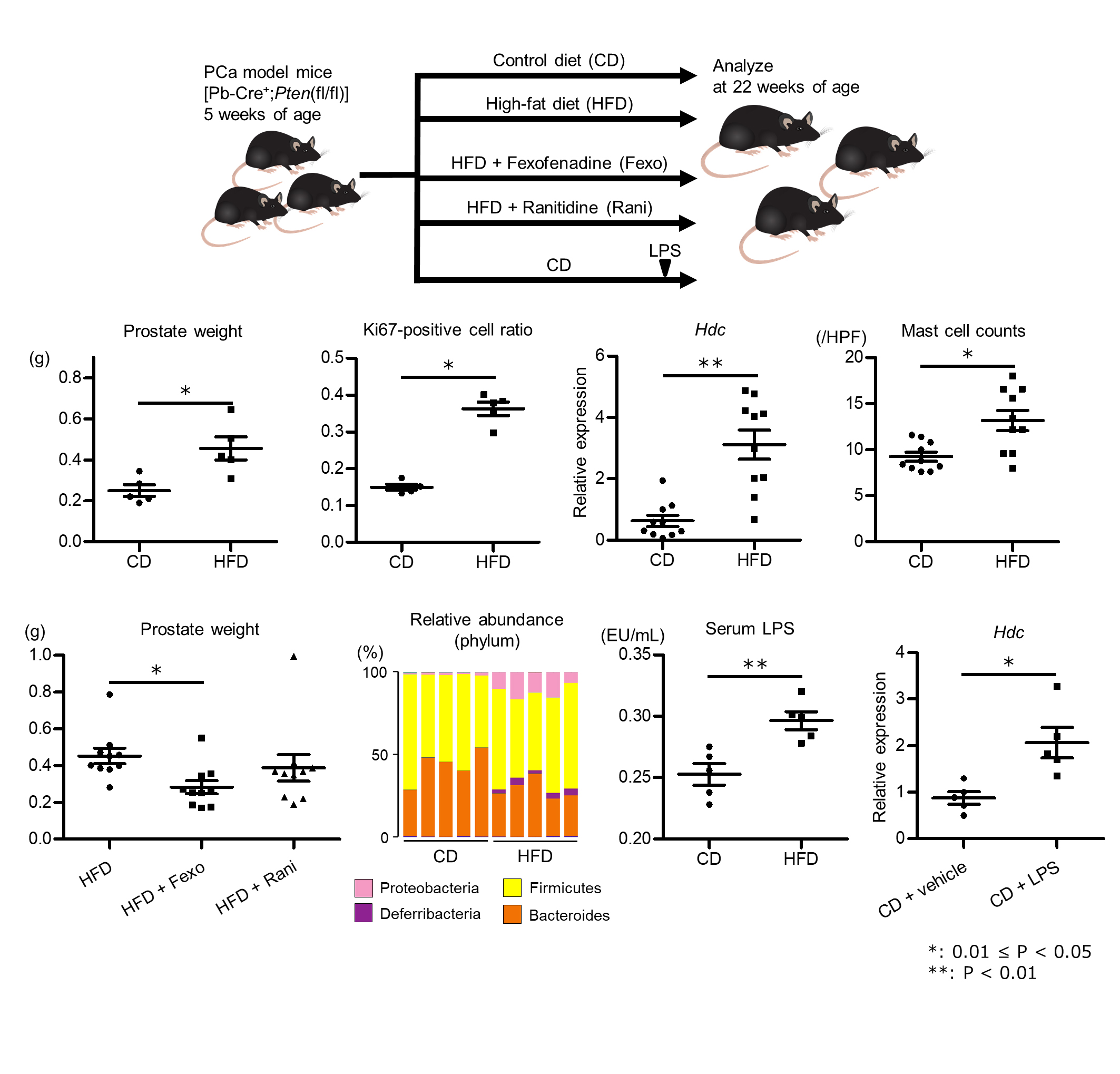
Methods: We compared between HFD-fed and control diet (CD)-fed prostate-speci?c Pten KO mice at 22 weeks of age as PCa models. HFD-fed mice were given histamine receptor antagonist. Tumor growth was evaluated by prostate weight and Ki67 staining. Gut microbiota compositions were analyzed by 16S rRNA gene sequencing of mouse feces. Histamine signaling in PCa was assessed at gene and protein level.
Results: HFD significantly increased prostate weight (0.25 vs. 0.46g, P = 0.01) and Ki67-positive cancer cell ratio (0.15 vs. 0.36, P = 0.01). cDNA microarray of PCa showed that expression of Hdc encoding histidine decarboxylase (HDC), which is responsible for histamine production, was upregulated in HFD-fed mice (Fold change = 2.71). The elevated expression of Hdc was also confirmed by quantitative PCR (P < 0.01). Infiltrating mast cells highly produced HDC protein, and mast cell counts around cancer foci were significantly increased in HFD-fed mice. Fexofenadine (Fexo), an H1 blocker, inhibited HFD-induced PCa growth, while ranitidine, an H2 blocker, had no inhibitory effect. Fexo reduced MDSC counts and suppressed IL6/STAT3 signaling in PCa of HFD-fed mice. In vitro, both histamine and Fexo did not affect the proliferation of DU145. HFD altered the composition of gut microbiota, and Shannon index suggesting diversity of gut microbiota was reduced in HFD-fed mice. Serum Lipopolysaccharide (LPS) levels were elevated in HFD-fed mice, and LPS injection resulted in upregulation of Hdc in PCa.
Conclusions: We found that HFD caused gut microbiota dysbiosis and elevated systemic LPS level, which could promote inflammatory PCa progression through histamine H1 receptor signaling. These results suggest the existence of a new LPS and histamine-mediated “gut-prostate axis” in addition to the IGF-1-mediated axis.
Source of Funding: This work was supported by research grants of The Japanese Urological Association.
Methods: We compared between HFD-fed and control diet (CD)-fed prostate-speci?c Pten KO mice at 22 weeks of age as PCa models. HFD-fed mice were given histamine receptor antagonist. Tumor growth was evaluated by prostate weight and Ki67 staining. Gut microbiota compositions were analyzed by 16S rRNA gene sequencing of mouse feces. Histamine signaling in PCa was assessed at gene and protein level.
Results: HFD significantly increased prostate weight (0.25 vs. 0.46g, P = 0.01) and Ki67-positive cancer cell ratio (0.15 vs. 0.36, P = 0.01). cDNA microarray of PCa showed that expression of Hdc encoding histidine decarboxylase (HDC), which is responsible for histamine production, was upregulated in HFD-fed mice (Fold change = 2.71). The elevated expression of Hdc was also confirmed by quantitative PCR (P < 0.01). Infiltrating mast cells highly produced HDC protein, and mast cell counts around cancer foci were significantly increased in HFD-fed mice. Fexofenadine (Fexo), an H1 blocker, inhibited HFD-induced PCa growth, while ranitidine, an H2 blocker, had no inhibitory effect. Fexo reduced MDSC counts and suppressed IL6/STAT3 signaling in PCa of HFD-fed mice. In vitro, both histamine and Fexo did not affect the proliferation of DU145. HFD altered the composition of gut microbiota, and Shannon index suggesting diversity of gut microbiota was reduced in HFD-fed mice. Serum Lipopolysaccharide (LPS) levels were elevated in HFD-fed mice, and LPS injection resulted in upregulation of Hdc in PCa.
Conclusions: We found that HFD caused gut microbiota dysbiosis and elevated systemic LPS level, which could promote inflammatory PCa progression through histamine H1 receptor signaling. These results suggest the existence of a new LPS and histamine-mediated “gut-prostate axis” in addition to the IGF-1-mediated axis.
Source of Funding: This work was supported by research grants of The Japanese Urological Association.


.jpg)
.jpg)