Back
Poster, Podium & Video Sessions
Podium
PD48: Pediatic Urology: Genitalia, Upper & Lower Urinary Tract
PD48-10: Testicular transcriptome analysis reveals potential genes associated with endocrine system in a mouse model of compensated testicular hypertrophy
Sunday, May 15, 2022
2:30 PM – 2:40 PM
Location: Room 244
Daniel E. Nassau*, Alexandra Dullea, Eliyahu Kresch, Vinayak Madhusoodanan, Shathiyah Kulandavelu, Miguel Castellan, Himanshu Arora, Ranjith Ramasamy, Miami, FL
Podium Presenter(s)
Introduction: Animal models demonstrate compensatory growth after unilateral orchiectomy when loss occurs before puberty, but absent if performed post-pubertally. The mechanisms leading to testicular hypertrophy after tissue loss remain unknown. We hypothesized that cellular pathways involving somatic growth and reproduction would be upregulated in the prepubertal groups compared to control. We performed RNA sequencing (RNASeq) on the contralateral testis after hemi-castration was performed at different ages in C57BL/6 mice.
Methods: An abdominal approach unilateral orchiectomy was performed in C57BL/6 mice at either the neonatal, prepubertal or post-pubertal periods between days of life (DOL) 2-4, 20-22 and 42-44, respectively. Experimental mice and controls were sacrificed after reaching physical maturity (DOL 80). The remaining testis was weighed and underwent RNASeq. The RNA library was generated and protein coding genes with p<0.05, FDR <0.05 were subjected to enrichment using Ingenuity pathway analysis (IPA). Expression of the candidate genes was evaluated using two-way ANOVA, allowing the identification of significantly different expression within the experimental groups compared to the control.
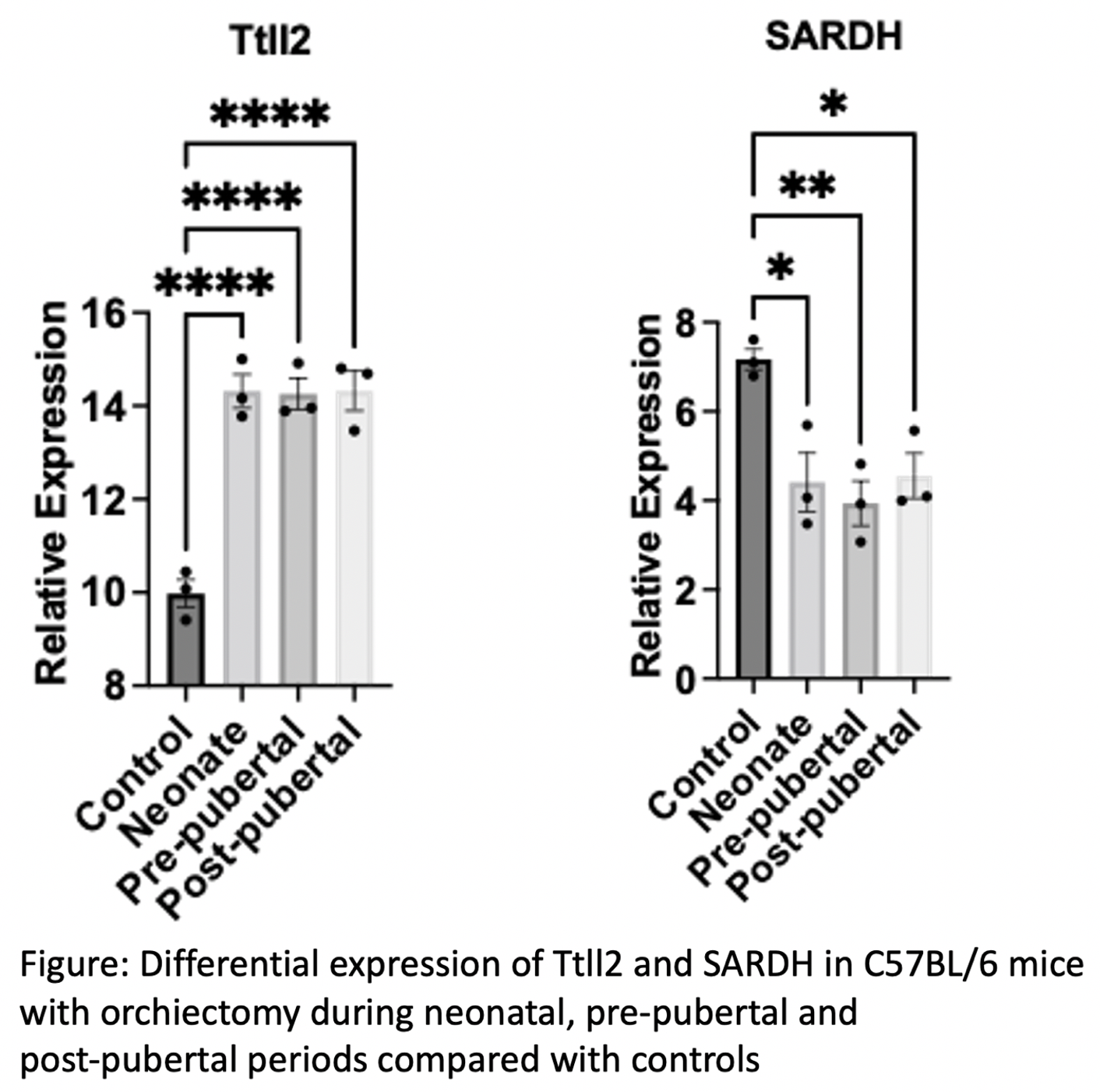
Results: Mean testis weight in each group (n=3) was 110.6mg (Neonate), 104.7mg (Prepubertal), 101.2 (Post-pubertal) and 85.5 (control) indicating larger testis on the contralateral side as an adult if orchiectomy was at an earlier age. IPA showed 502 different pathways/functions with which candidate genes were involved. A total of 58 unique protein coding genes met the criteria for analysis. Of these, Tubulin Tyrosine Ligase Like 2 (Ttll2) and Sacrosine Dehydrogenase (SARDH) had significantly increased, and decreased expression compared with controls, respectively (Figure). Ttll2 and SARDH are enriched in pathways such as endocrine system disorder, reproductive system disease, developmental disorders and metabolic diseases.
Conclusions: We identified two genes involved in endocrine pathways that likely have altered expression in response to hemi-castration. Further work is needed to understand how these genes may be involved in testicular growth, and how pre-pubertal testis loss influences hypertrophy as compared to loss of testis as an adult.
Source of Funding: This work was supported by National Institutes of Health Grant R01 DK130991, and Clinician Scientist Development Grant from the American Cancer Society to Ranjith Ramasamy
Methods: An abdominal approach unilateral orchiectomy was performed in C57BL/6 mice at either the neonatal, prepubertal or post-pubertal periods between days of life (DOL) 2-4, 20-22 and 42-44, respectively. Experimental mice and controls were sacrificed after reaching physical maturity (DOL 80). The remaining testis was weighed and underwent RNASeq. The RNA library was generated and protein coding genes with p<0.05, FDR <0.05 were subjected to enrichment using Ingenuity pathway analysis (IPA). Expression of the candidate genes was evaluated using two-way ANOVA, allowing the identification of significantly different expression within the experimental groups compared to the control.
Results: Mean testis weight in each group (n=3) was 110.6mg (Neonate), 104.7mg (Prepubertal), 101.2 (Post-pubertal) and 85.5 (control) indicating larger testis on the contralateral side as an adult if orchiectomy was at an earlier age. IPA showed 502 different pathways/functions with which candidate genes were involved. A total of 58 unique protein coding genes met the criteria for analysis. Of these, Tubulin Tyrosine Ligase Like 2 (Ttll2) and Sacrosine Dehydrogenase (SARDH) had significantly increased, and decreased expression compared with controls, respectively (Figure). Ttll2 and SARDH are enriched in pathways such as endocrine system disorder, reproductive system disease, developmental disorders and metabolic diseases.
Conclusions: We identified two genes involved in endocrine pathways that likely have altered expression in response to hemi-castration. Further work is needed to understand how these genes may be involved in testicular growth, and how pre-pubertal testis loss influences hypertrophy as compared to loss of testis as an adult.
Source of Funding: This work was supported by National Institutes of Health Grant R01 DK130991, and Clinician Scientist Development Grant from the American Cancer Society to Ranjith Ramasamy


.jpg)
.jpg)
