Back
Poster, Podium & Video Sessions
Podium
PD50: Stone Disease: Epidemiology & Evaluation II
PD50-07: Defining Stone Passage in a Clinical Trial: Adjudication in the Prevention of Urinary Stones with Hydration (PUSH) Study
Sunday, May 15, 2022
4:30 PM – 4:40 PM
Location: Room 255
Hunter Wessells, Seattle, WA, Henry Lai, St. Louis, MO, John Lieske*, Rochester, MN, Brian Matlaga, Baltimore, MD, Sharon Settles, Hongqiu Yang, Durham, NC, Jonathan Harper, Seattle, WA, Charles Scales Jr, Durham, NC, Alana Desai, St. Louis, MO, Naim Maalouf, Dallas, TX, Peter Reese, Sri Sivalingam, Durham, NC, Gregory Tasian, Philadelphia, PA, Hussein Al-Khalidi, Durham, NC, Ziya Kirkali, Bethesda, MD

John Charles Lieske, MD
Mayo Clinic
Podium Presenter(s)
Introduction: Clinical trials in patients with urinary stone disease have used variable definitions of stone events/ passage. We assessed the accuracy of self-reported stone passage in a large clinical trial using a blinded adjudication process.
Methods: Study participants (n=1642) in the two arm PUSH trial are randomized either to a behavioral intervention or control arm to increase and maintain high fluid intake. The primary endpoint is symptomatic kidney stone recurrence including stone passage observed and/or captured by the participant or procedural intervention for stones. Adjudication of the primary endpoint (Confirmed Event) requires corroboration via photograph, stone specimen, imaging, definitive record of symptoms (pain, nausea/vomiting, urinary symptoms, hematuria) and passage and/or surgical intervention in clinical notes. A Suspected Stone Event (SSE) category was created for participants with symptoms and passage without complete documentation.
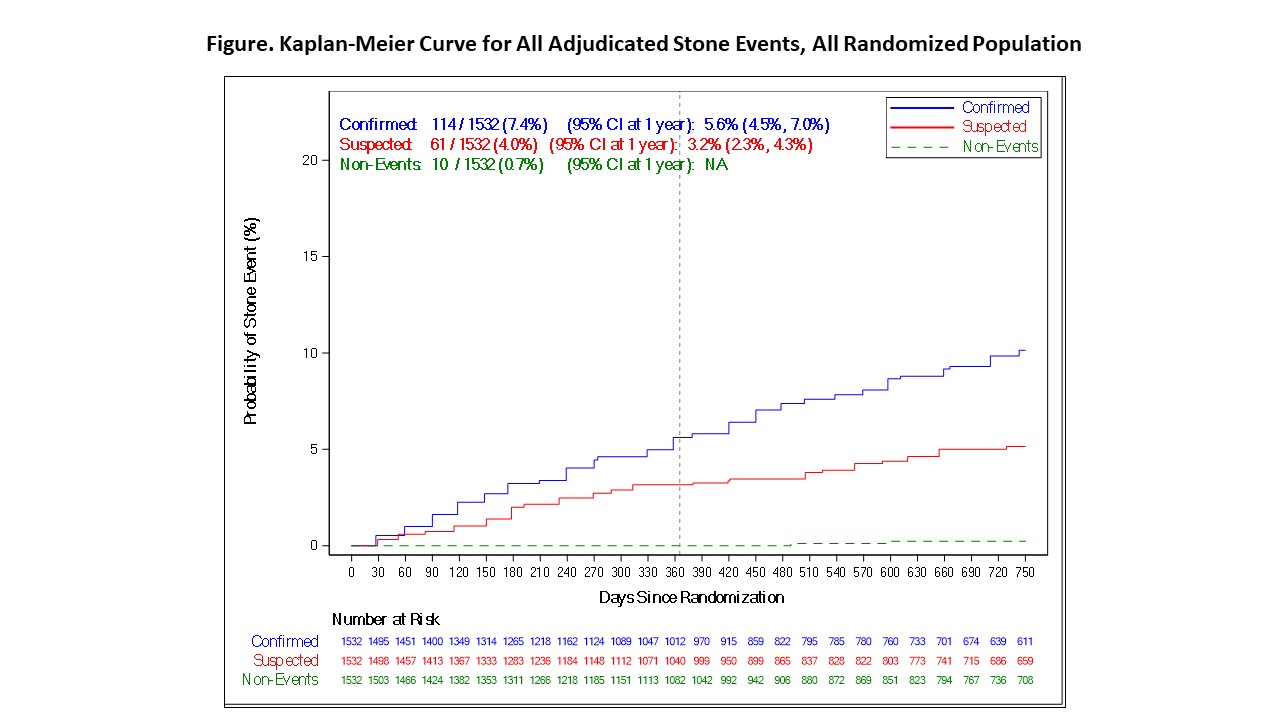
Results: At time of this analysis 1532 participants were randomized with a median of 692 days of follow-up. Self-reported stone passage (n=185) was adjudicated by an independent committee as confirmed in 114 (61.6%), SSE in 61 (33%), and non-events in 10 (5.4%). The overall Kaplan Meier rate of confirmed plus suspected events at 1 year was 9.1% (95% CI 7.7-10.8). The rates of adjudicated stone passage varied across the duration of the study (Figure).
Conclusions: In the PUSH trial rigorous blinded adjudication revealed that self-reported stone passage overwhelmingly represented definitive or likely (SSE) events at all time points up to 2 years. The divergence of the rate of confirmed vs suspected events after one year requires further investigation. The inclusion of SSE and procedural intervention allows for the full burden of stone events to be ascertained. These results will inform endpoint assessment in future urinary stone trials.
Source of Funding: NIH/NIDDK
Methods: Study participants (n=1642) in the two arm PUSH trial are randomized either to a behavioral intervention or control arm to increase and maintain high fluid intake. The primary endpoint is symptomatic kidney stone recurrence including stone passage observed and/or captured by the participant or procedural intervention for stones. Adjudication of the primary endpoint (Confirmed Event) requires corroboration via photograph, stone specimen, imaging, definitive record of symptoms (pain, nausea/vomiting, urinary symptoms, hematuria) and passage and/or surgical intervention in clinical notes. A Suspected Stone Event (SSE) category was created for participants with symptoms and passage without complete documentation.
Results: At time of this analysis 1532 participants were randomized with a median of 692 days of follow-up. Self-reported stone passage (n=185) was adjudicated by an independent committee as confirmed in 114 (61.6%), SSE in 61 (33%), and non-events in 10 (5.4%). The overall Kaplan Meier rate of confirmed plus suspected events at 1 year was 9.1% (95% CI 7.7-10.8). The rates of adjudicated stone passage varied across the duration of the study (Figure).
Conclusions: In the PUSH trial rigorous blinded adjudication revealed that self-reported stone passage overwhelmingly represented definitive or likely (SSE) events at all time points up to 2 years. The divergence of the rate of confirmed vs suspected events after one year requires further investigation. The inclusion of SSE and procedural intervention allows for the full burden of stone events to be ascertained. These results will inform endpoint assessment in future urinary stone trials.
Source of Funding: NIH/NIDDK


.jpg)
.jpg)