Back
Poster, Podium & Video Sessions
Podium
PD52: Sexual Function/Dysfunction: Surgical Therapy II
PD52-05: Uninfected inflatable penile prostheses uniformly demonstrate rich dynamic bacterial biofilms with complex underlying microbe-metabolite interaction networks
Monday, May 16, 2022
7:40 AM – 7:50 AM
Location: Room 252
Glenn T. Werneburg, Daniel Hettel*, Bradley Gill, Ava Adler, Hadley Wood, Petar Bajic, Kenneth Angermeier, Scott Lundy, Daniel Shoskes, Aaron Miller, Cleveland, OH
- DH
Daniel Hettel, MD
Cleveland Clinic
Podium Presenter(s)
Introduction: The inflatable penile prosthesis (IPP) is an effective erectile dysfunction treatment, but a subset of patients will require device removal due to infection. To understand the transition from colonized (wherein microbes present but not pathologic) to infected states, we sought to determine uninfected IPP biofilm microbial and metabolite composition, interaction networks, and clinical factor association. We hypothesized interaction networks would identify species and pathways within the IPP biofilm community, which may protect against infection.
Methods: Patients scheduled for IPP removal/revision consented per IRB-approved protocol. Devices were swabbed upon first access with safeguards to avoid contamination. Samples, with subcutaneous controls, underwent next-generation sequencing and metabolomics. Data integration generated interaction networks. Biofilm diversity association with clinical factors was analyzed with t-tests and ANOVA. Isolates were cultured from devices and biofilm formation was reconstituted in vitro and assessed.
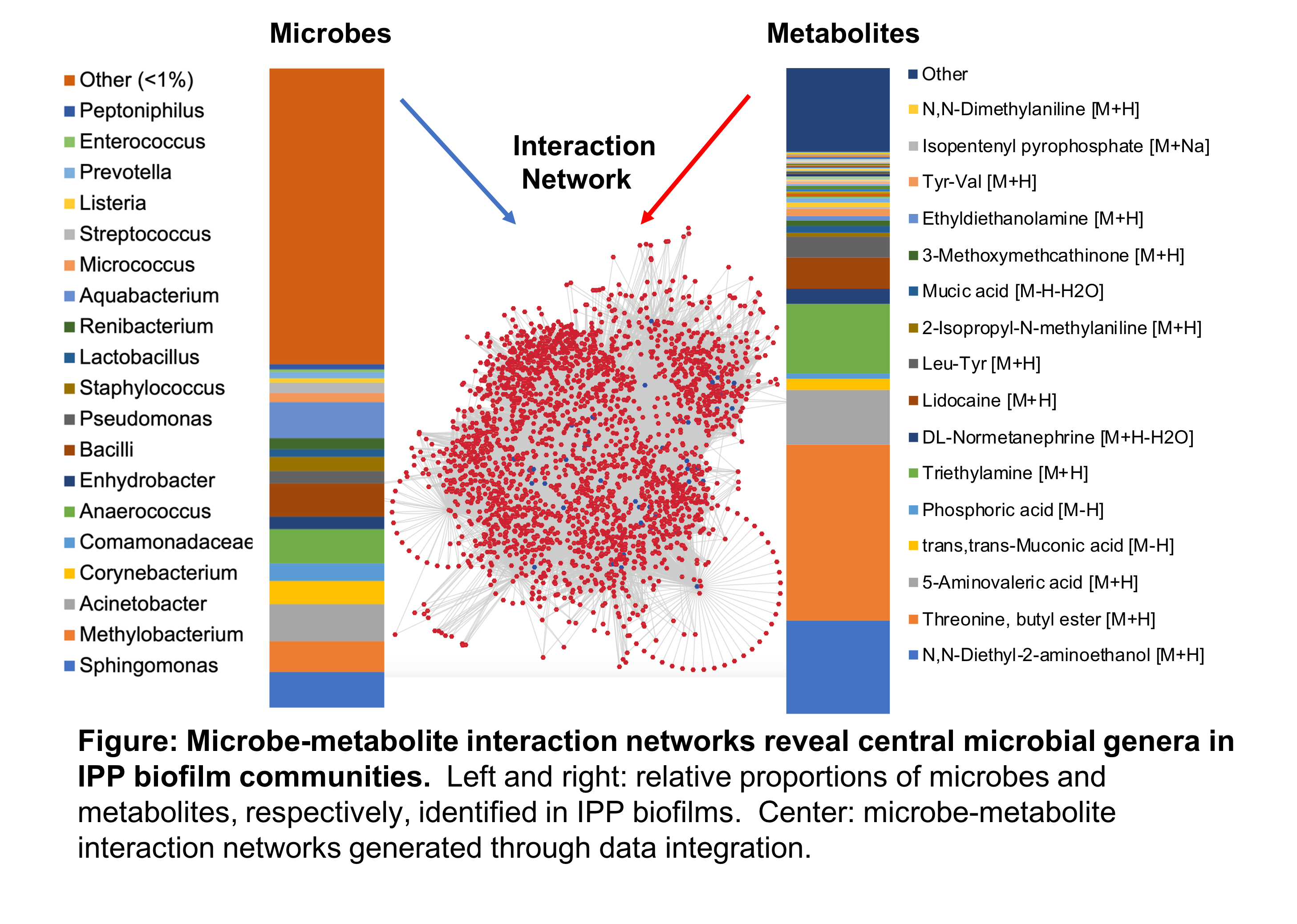
Results: All 20 uninfected devices studied harbored microbiota. Most common genera included Acinetobacter, Aquabacterium, Sphingomonas, and Anaerococcus (Figure left). Diversity increased with indwelling time (p=0.025), but did not differ by age, recent antibiotics, or BMI. Threonine-ester (peptide precursor), diethanolamine (anesthetic precursor), and triethylamine were the most common metabolites (Figure right). Central genera in the microbe-metabolite interaction networks included Anaerococcus, Aquabacterium, Staphylococcus, and Pseudomonas (Figure center). Biofilm formation of Staphylococcus epidermidis isolated from an IPP, was successfully re-constituted in vitro and quantified.
Conclusions: Microbe-metabolite interaction networks revealed a combination of putative commensal, opportunistic, and pathogenic genera of central metabolic importance. A device-associated pathogen was isolated and identified, and biofilm formation was reconstituted. Our results open new avenues to investigate biochemical changes in transition from colonization to clinical infection, and identify preventive strategies including novel device materials and coatings.
Source of Funding: N/A
Methods: Patients scheduled for IPP removal/revision consented per IRB-approved protocol. Devices were swabbed upon first access with safeguards to avoid contamination. Samples, with subcutaneous controls, underwent next-generation sequencing and metabolomics. Data integration generated interaction networks. Biofilm diversity association with clinical factors was analyzed with t-tests and ANOVA. Isolates were cultured from devices and biofilm formation was reconstituted in vitro and assessed.
Results: All 20 uninfected devices studied harbored microbiota. Most common genera included Acinetobacter, Aquabacterium, Sphingomonas, and Anaerococcus (Figure left). Diversity increased with indwelling time (p=0.025), but did not differ by age, recent antibiotics, or BMI. Threonine-ester (peptide precursor), diethanolamine (anesthetic precursor), and triethylamine were the most common metabolites (Figure right). Central genera in the microbe-metabolite interaction networks included Anaerococcus, Aquabacterium, Staphylococcus, and Pseudomonas (Figure center). Biofilm formation of Staphylococcus epidermidis isolated from an IPP, was successfully re-constituted in vitro and quantified.
Conclusions: Microbe-metabolite interaction networks revealed a combination of putative commensal, opportunistic, and pathogenic genera of central metabolic importance. A device-associated pathogen was isolated and identified, and biofilm formation was reconstituted. Our results open new avenues to investigate biochemical changes in transition from colonization to clinical infection, and identify preventive strategies including novel device materials and coatings.
Source of Funding: N/A


.jpg)
.jpg)