Back
Poster, Podium & Video Sessions
Podium
PD53: Bladder Cancer: Basic Research & Pathophysiology III
PD53-09: Antibody drug conjugates and variant histology muscle-invasive bladder cancer: Are the targets present in primary and/or metastatic tumors?
Monday, May 16, 2022
8:20 AM – 8:30 AM
Location: Room 255
Fady Ghali*, Martine Roudier, Funda Vakar-Lopez, Jose Garcia, Yan Wang, Gavin Ha, Petros Grivas, John Lee, Evan Yu, Bruce Montgomery, Andrew Hsieh, Jonathan Wright, Hung-Ming Lam, Seattle, WA

Fady Ghali, MD
MD
University of Washington
Podium Presenter(s)
Introduction: Nectin-4 and Trop-2 are cell surface targets of novel humanized monoclonal antibody-drug conjugates, Enfortumab-vedotin (EV) and Sacituzumab govitecan (SG), respectively. These agents are FDA-approved for locally advanced/metastatic urothelial cancer (UC), however their wider role in the treatment of variant histology bladder cancer (BC) is unknown. We describe the expression level and sub-cellular localization of Nectin-4 and TROP-2 via immunostaining in histologic variants of BC within primary and matched metastatic samples.
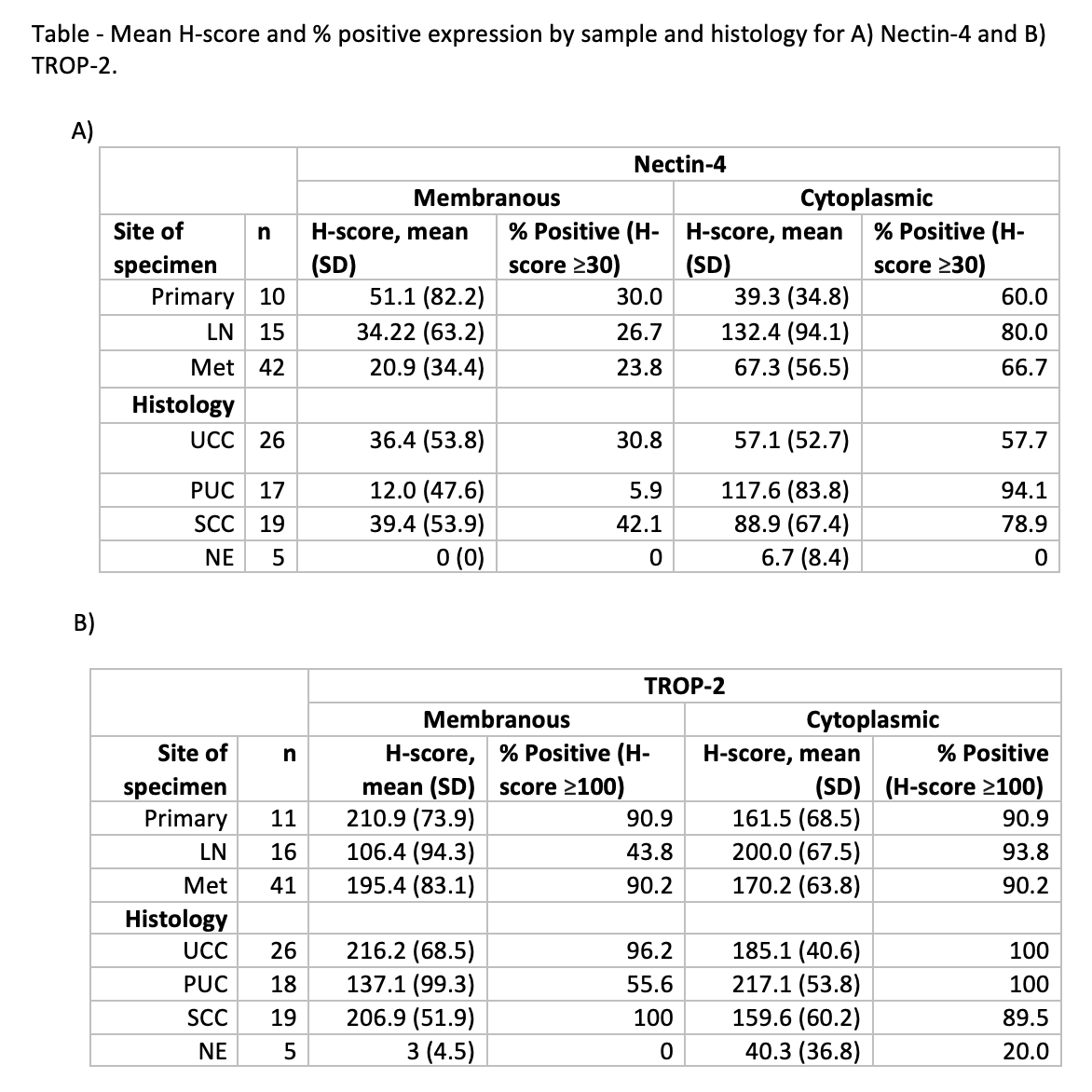
Methods: Immunohistochemistry for Nectin-4 and TROP-2 was performed (using Abcam 192033 and 214488 respectively) on matched primary and metastatic tumor samples from patients with metastatic BC collected via rapid autopsy. Staining was scored by intensity (0-3) and multiplied by % of positive cells resulting in a range of 0-300 H-score. Membranous and cytoplasmic staining were separately evaluated for each sample.
Results: A total of 68 samples from 20 patients were collected and analyzed, of which 27 were urothelial carcinoma (UC), 17 plasmacytoid (PUC), 19 Squamous cell carcinoma (SCC), and 5 neuroendocrine (NE); 11 samples were collected from primary tumor sites, 20 from lymph node (LN) and 37 from various metastatic sites; 17 of 20 patients (85%) had received systemic chemotherapy and 35% had received immunotherapy. No patient had received EV or SG. Table 1 demonstrates mean membranous and cytoplasmic H-score levels as well as % positive rates for Nectin-4 and TROP-2. Nectin-4 localized primarily in the cytoplasm rather than the membrane in PUC and SCC for LN and metastatic sites, while primary tumors demonstrated relatively even Nectin-4 distribution between membrane and cytoplasm. TROP-2 was expressed at equivalent levels in both membrane and cytoplasm for both PUC and SCC, and the high expression was conserved from primary to metastases.
Conclusions: Nectin-4 and TROP-2 are highly expressed in UC and variant histology BC, both at primary and metastatic sites, but not in NE histology. Whether differences in membranous vs cytoplasmic localization affects response to EV or SG is unknown. The implications of these findings on treatment response for variant histology BC warrant further study in clinical trials, which we are launching.
Source of Funding: N/A
Methods: Immunohistochemistry for Nectin-4 and TROP-2 was performed (using Abcam 192033 and 214488 respectively) on matched primary and metastatic tumor samples from patients with metastatic BC collected via rapid autopsy. Staining was scored by intensity (0-3) and multiplied by % of positive cells resulting in a range of 0-300 H-score. Membranous and cytoplasmic staining were separately evaluated for each sample.
Results: A total of 68 samples from 20 patients were collected and analyzed, of which 27 were urothelial carcinoma (UC), 17 plasmacytoid (PUC), 19 Squamous cell carcinoma (SCC), and 5 neuroendocrine (NE); 11 samples were collected from primary tumor sites, 20 from lymph node (LN) and 37 from various metastatic sites; 17 of 20 patients (85%) had received systemic chemotherapy and 35% had received immunotherapy. No patient had received EV or SG. Table 1 demonstrates mean membranous and cytoplasmic H-score levels as well as % positive rates for Nectin-4 and TROP-2. Nectin-4 localized primarily in the cytoplasm rather than the membrane in PUC and SCC for LN and metastatic sites, while primary tumors demonstrated relatively even Nectin-4 distribution between membrane and cytoplasm. TROP-2 was expressed at equivalent levels in both membrane and cytoplasm for both PUC and SCC, and the high expression was conserved from primary to metastases.
Conclusions: Nectin-4 and TROP-2 are highly expressed in UC and variant histology BC, both at primary and metastatic sites, but not in NE histology. Whether differences in membranous vs cytoplasmic localization affects response to EV or SG is unknown. The implications of these findings on treatment response for variant histology BC warrant further study in clinical trials, which we are launching.
Source of Funding: N/A


.jpg)
.jpg)