Back
Poster, Podium & Video Sessions
Podium
PD59: Kidney Cancer: Advanced (including Drug Therapy) II
PD59-04: Application of the KEYNOTE-564 trial results to real life population: which patients with advanced kidney cancer should receive adjuvant pembrolizumab
Monday, May 16, 2022
1:30 PM – 1:40 PM
Location: Room 255
Giuseppe Fallara*, Giuseppe Rosiello, Daniele Raggi, Laura Marandino, Greta Malena, Giuseppe Basile, Gianmarco Colandrea, Daniele Cignoli, Federico Belladelli, Giacomo Musso, Francesco Cei, Roberto Bertini, Alberto Briganti, Andrea Salonia, Francesco Montorsi, Alessandro Larcher, Andrea Necchi, Umberto Capitanio, Milan, Italy

Giuseppe Fallara, MD
Vita-Salute San Raffaele University Milan
Podium Presenter(s)
Introduction: The KEYNOTE-564 has reported improved disease-free survival (DFS) for high-risk renal cell carcinoma (RCC) patients receiving adjuvant Pembrolizumab when compared with placebo. However, given toxicity, costs, and heterogeneity in survival outcomes, it is of utmost importance an accurate selection of those patients who may benefit from adjuvant Pembrolizumab.
Methods: From our institutional prospectively maintained database we identified 408 patients who met the KEYNOTE-564 inclusion criteria (pT2 with grade 4 and/or sarcomatoid differentiation, pT3-4, regional lymph-node metastasis (LNI) or stage M1 with NED and the following features: ECOG 0-1, no bone or brain metastases and no previous neoadjuvant therapies). Descriptive statistics compared our cohort to the placebo arm of the KEYNOTE-564 and Kaplan-Meier analyses were used to assess time to DFS. We then applied a regression tree method for censored data to generate a risk stratification tool to predict 12-months DFS.
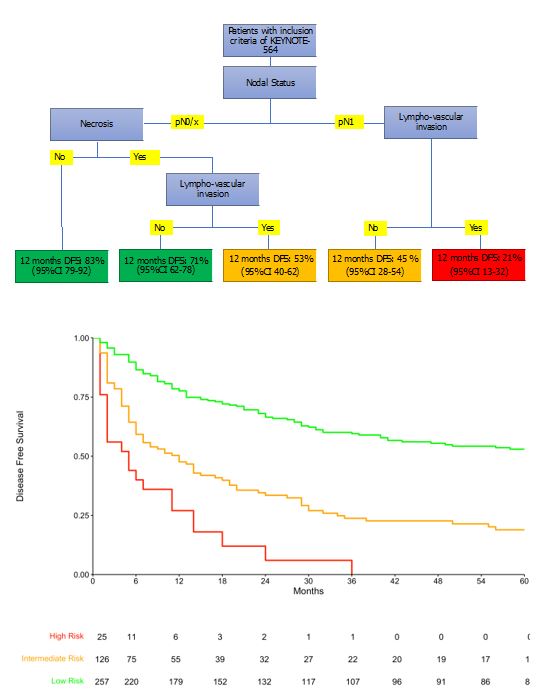
Results: Relative to the trial population, in the real-life setting patients had worst ECOG (ECOG=1, 64 vs. 15%) and in 44% of cases had one or more comorbidity, despite similar age at baseline. Similarly, there were more pT4 (5.4 vs. 2.7%) and pN1 (15 vs 6.3%) cases. Median DFS was 29 months (95%CI 23-37) compared to median DFS not reached in the KEYNOTE-564. According to regression tree analysis, LNI and lympho-vascular invasion (LVI) identified patients at higher risk of 1-yr disease recurrence (1-yr DFS 21%); those with LNI and LVI- and those pN0/x and necrosis and LVI at pathological report were at intermediate risk (1-yr DFS 51%); those with pN0/x with necrosis but without LVI and those with pN0/X without necrosis were at low risk of recurrence (1-yr DFS 79%). Our study is limited by potential unmeasured confounders and by lack of validation.
Conclusions: Patients eligible for adjuvant Pembrolizumab have significantly different baseline characteristics, pathological features, and early recurrence outcomes rates relative to the placebo arm of KEYNOTE-564. This implies the need to accurately identify patients who may benefit the most from the adjuvant regimen. Those with necrosis, LVI invasion and positive nodes appear those who might benefit the most from adjuvant treatment.
Source of Funding: no
Methods: From our institutional prospectively maintained database we identified 408 patients who met the KEYNOTE-564 inclusion criteria (pT2 with grade 4 and/or sarcomatoid differentiation, pT3-4, regional lymph-node metastasis (LNI) or stage M1 with NED and the following features: ECOG 0-1, no bone or brain metastases and no previous neoadjuvant therapies). Descriptive statistics compared our cohort to the placebo arm of the KEYNOTE-564 and Kaplan-Meier analyses were used to assess time to DFS. We then applied a regression tree method for censored data to generate a risk stratification tool to predict 12-months DFS.
Results: Relative to the trial population, in the real-life setting patients had worst ECOG (ECOG=1, 64 vs. 15%) and in 44% of cases had one or more comorbidity, despite similar age at baseline. Similarly, there were more pT4 (5.4 vs. 2.7%) and pN1 (15 vs 6.3%) cases. Median DFS was 29 months (95%CI 23-37) compared to median DFS not reached in the KEYNOTE-564. According to regression tree analysis, LNI and lympho-vascular invasion (LVI) identified patients at higher risk of 1-yr disease recurrence (1-yr DFS 21%); those with LNI and LVI- and those pN0/x and necrosis and LVI at pathological report were at intermediate risk (1-yr DFS 51%); those with pN0/x with necrosis but without LVI and those with pN0/X without necrosis were at low risk of recurrence (1-yr DFS 79%). Our study is limited by potential unmeasured confounders and by lack of validation.
Conclusions: Patients eligible for adjuvant Pembrolizumab have significantly different baseline characteristics, pathological features, and early recurrence outcomes rates relative to the placebo arm of KEYNOTE-564. This implies the need to accurately identify patients who may benefit the most from the adjuvant regimen. Those with necrosis, LVI invasion and positive nodes appear those who might benefit the most from adjuvant treatment.
Source of Funding: no

.jpg)
.jpg)