Back
Poster, Podium & Video Sessions
Podium
PD59: Kidney Cancer: Advanced (including Drug Therapy) II
PD59-06: Pembrolizumab outperforms tyrosine kinase inhibitors in the adjuvant therapy of patients with high-risk renal cell carcinoma: a systematic review and network meta-analysis
Monday, May 16, 2022
1:50 PM – 2:00 PM
Location: Room 255
Ekaterina Laukhtina*, Fahad Quhal, Keiichiro Mori, Reza Sari Motlagh, Satoshi Katayama, Pawel Rajwa, Takafumi Yanagisawa, Nico С. Grossmann, Hadi Mostafaei, Frederik König, Abdulmajeed Aydh, Benjamin Pradere, Vienna, Austria, Jeremy Yuen-Chun Teoh, Hong Kon, China, People's Republic of, Dmitry Enikeev, Moscow, Russian Federation, Pierre I. Karakiewicz, Montreal, Canada, Manuela Schmidinger, Shahrokh F. Shariat, Vienna, Austria

Ekaterina Laukhtina
Medical University of Vienna
Podium Presenter(s)
Introduction: Immune checkpoint inhibitors (ICIs) are effective in the management of renal cell carcinoma (RCC) becoming the standard of care in metastatic RCC patients. Usage of these agents in the adjuvant setting may improve the prognosis of patients with non-metastatic high risk RCC. This study aimed to determine the oncological outcomes and safety profiles of adjuvant ICIs compared to adjuvant TKIs in patients with non-metastatic RCC after surgical extirpation.
Methods: The MEDLINE and EMBASE databases were searched to identify phase III randomized controlled trials reporting on oncologic and toxicity outcomes of adjuvant ICIs and TKIs in RCC patients. The primary outcomes of interest were disease-free survival (DFS) and overall survival (OS), and the secondary outcomes were adverse events (AEs). Network meta-analyses (NMA) were performed of different therapy regimens with placebo as the common comparator arm.
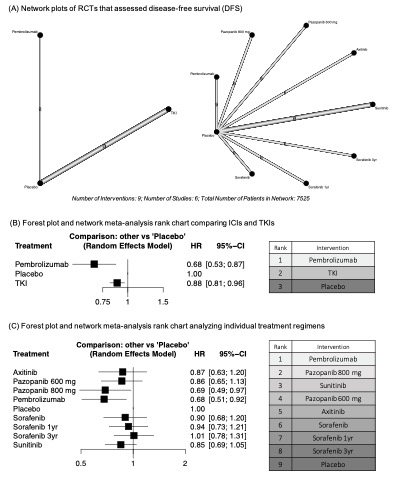
Results: Six trials (KEYNOTE-564, S-TRAC, ASSURE, PROTECT, ATLAS, and SORCE) were included in our analysis. Compared to placebo, both pembrolizumab (HR: 0.68, 95%CrI: 0.51–0.92) and pazopanib 800 mg (HR: 0.69, 95%CrI: 0.49–0.97) were significantly associated with improved DFS. Adjuvant pembrolizumab (HR: 0.54, 95%CrI: 0.30–0.97) was significantly associated with an improved OS compared to TKIs (HR: 0.93, 95%CrI: 0.83–1.04). Based on the analysis of treatment ranking, pembrolizumab appeared as the best treatment with regards to both DFS and OS. Although pembrolizumab resulted in significantly higher rate of AEs compared to placebo, based on the analysis of treatment ranking, pembrolizumab had the lower likelihood of any and high-grade AEs compared to TKIs.
Conclusions: Our analysis indicates the superior oncologic benefit of adjuvant pembrolizumab in patients with non-metastatic RCC after surgical extirpation compared to those receiving adjuvant TKIs. This together with the significant toxicity of TKIs therapy, supports pembrolizumab as the new standard of care in the adjuvant setting for RCC patients at high risk of relapse.
Source of Funding: None.
Methods: The MEDLINE and EMBASE databases were searched to identify phase III randomized controlled trials reporting on oncologic and toxicity outcomes of adjuvant ICIs and TKIs in RCC patients. The primary outcomes of interest were disease-free survival (DFS) and overall survival (OS), and the secondary outcomes were adverse events (AEs). Network meta-analyses (NMA) were performed of different therapy regimens with placebo as the common comparator arm.
Results: Six trials (KEYNOTE-564, S-TRAC, ASSURE, PROTECT, ATLAS, and SORCE) were included in our analysis. Compared to placebo, both pembrolizumab (HR: 0.68, 95%CrI: 0.51–0.92) and pazopanib 800 mg (HR: 0.69, 95%CrI: 0.49–0.97) were significantly associated with improved DFS. Adjuvant pembrolizumab (HR: 0.54, 95%CrI: 0.30–0.97) was significantly associated with an improved OS compared to TKIs (HR: 0.93, 95%CrI: 0.83–1.04). Based on the analysis of treatment ranking, pembrolizumab appeared as the best treatment with regards to both DFS and OS. Although pembrolizumab resulted in significantly higher rate of AEs compared to placebo, based on the analysis of treatment ranking, pembrolizumab had the lower likelihood of any and high-grade AEs compared to TKIs.
Conclusions: Our analysis indicates the superior oncologic benefit of adjuvant pembrolizumab in patients with non-metastatic RCC after surgical extirpation compared to those receiving adjuvant TKIs. This together with the significant toxicity of TKIs therapy, supports pembrolizumab as the new standard of care in the adjuvant setting for RCC patients at high risk of relapse.
Source of Funding: None.


.jpg)
.jpg)