Back
Poster, Podium & Video Sessions
Late-breaking Abstract I - Cancer & BPH
LBA01-06: Long term follow-up of an international multicenter prospective study in application of temporary implantable nitinol device (iTind) in men with lower urinary tract symptoms for BPH
Sunday, May 15, 2022
1:50 PM – 2:00 PM
Location: Room 243
Daniele A, Sabrina De Cillis, Cristian Fiori, Gregor Kadner, Claude Schulman, Francesco Porpiglia
.jpg)
Daniele Amparore, PhD,FEBU
University of Turin
Podium Presenter(s)
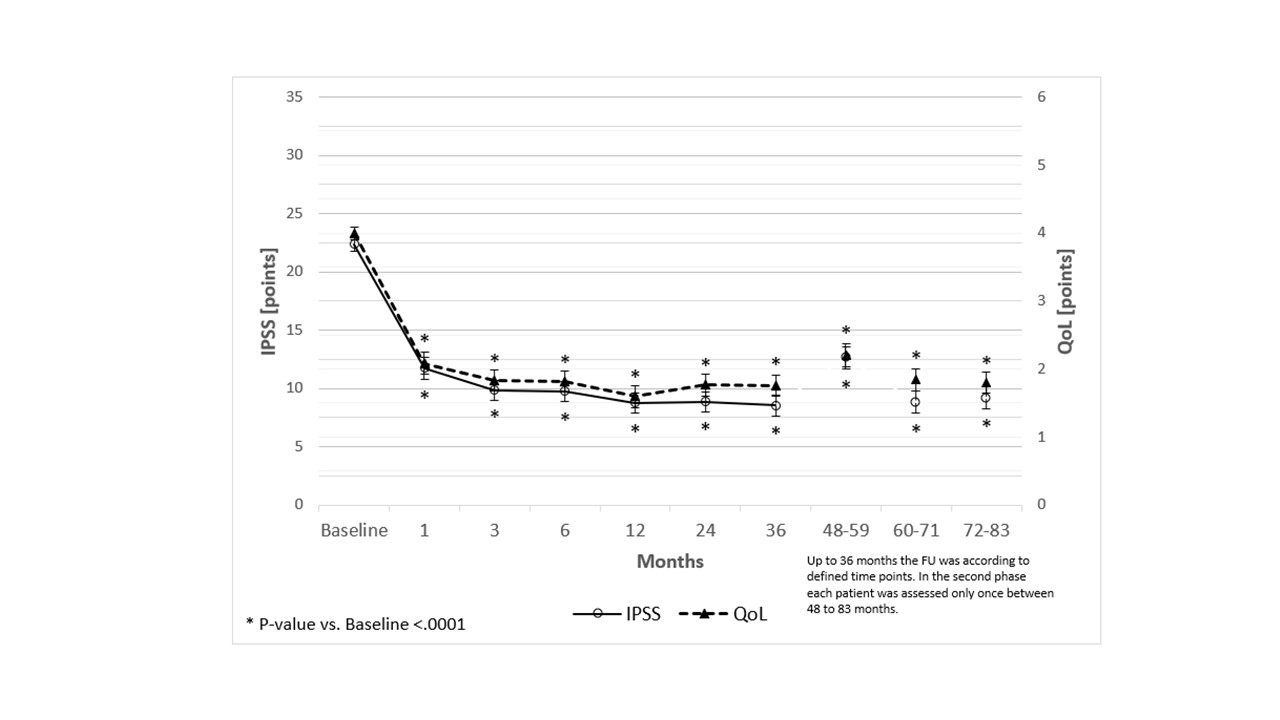
Introduction: To report IPSS, QoL and treatment failure rate up to 79-months for the MT02 study with implantation of the temporary implantable nitinol device (iTind; Medi-Tate Ltd®, Israel) in men with lower urinary tract symptoms (LUTS) due to benign prostatic obstruction (BPO).
Methods: Three out of nine international centers consented to continue the international prospective multicenter study on patients with LUTS due to BPO (IPSS ≥10, Qmax
Results: Fifty to 79 months results were available for 42 patients. Four patients were lost to follow-up and two patients deceased from reasons unrelated to the iTind device. Only two patients had treatment failures (one patient underwent TURP, the other ThuLEP), while no patient required any additional medication. IPSS average results were 12.63±8.84 (50-59m, N=24), 8.85 ±5.54 (60-71m, N=13) and 9.20 ±5.85 (72-79m, N=5). QoL average results were 2.21 ±1.69 (50-59m, N=24), 1.85 ±0.99 (60-71m, N=13) and 1.80 ±1.10 (72-79m, N=5). IPSS (-8.88, -10.31, and -9.60) and IPSS-QoL (-2.04, -1.85 and -1.80) improved significantly for all groups vs. baseline, respectively (p
Conclusions: iTind for treatment of LUTS secondary to BPO is an effective and safe procedure providing significant and effective reduction in symptoms and quality of life durable up to 79 months (6.6 years) with only 4% of treatment failures after 3-year follow-up.
Source of Funding: Olympus Corporation of the Americas (former Medi-Tate) funded this study.
Methods: Three out of nine international centers consented to continue the international prospective multicenter study on patients with LUTS due to BPO (IPSS ≥10, Qmax
Results: Fifty to 79 months results were available for 42 patients. Four patients were lost to follow-up and two patients deceased from reasons unrelated to the iTind device. Only two patients had treatment failures (one patient underwent TURP, the other ThuLEP), while no patient required any additional medication. IPSS average results were 12.63±8.84 (50-59m, N=24), 8.85 ±5.54 (60-71m, N=13) and 9.20 ±5.85 (72-79m, N=5). QoL average results were 2.21 ±1.69 (50-59m, N=24), 1.85 ±0.99 (60-71m, N=13) and 1.80 ±1.10 (72-79m, N=5). IPSS (-8.88, -10.31, and -9.60) and IPSS-QoL (-2.04, -1.85 and -1.80) improved significantly for all groups vs. baseline, respectively (p
Conclusions: iTind for treatment of LUTS secondary to BPO is an effective and safe procedure providing significant and effective reduction in symptoms and quality of life durable up to 79 months (6.6 years) with only 4% of treatment failures after 3-year follow-up.
Source of Funding: Olympus Corporation of the Americas (former Medi-Tate) funded this study.


.jpg)
.jpg)