Back
Poster, Podium & Video Sessions
Late-breaking Abstract II - Benign Disease
LBA02-02: Development of a Multiphasic Bladder Patch Composed of Collagen, Amniotic Membrane, and Patient-Derived Epithelial Cells
Sunday, May 15, 2022
3:40 PM – 3:50 PM
Location: Room 243
Ilaha Isali, Phillip McClellan, Thomas Wong, Ozan Akkus, Rachel Pope, Shubham Gupta, Adonis Hijaz
- II
Ilaha Isali, MD
Case Western Reserve University
Podium Presenter(s)
Introduction: Replacing bladder tissue with a functional equivalent remains one of the most challenging problems for conditions such as iatrogenic injuries, trauma, or fistula in reconstructive urology. Our study objective was to develop a bioengineered regenerative cell-seeded multiphasic bladder patch for bladder reconstruction.
Methods: Human oral epithelial cells were harvested from buccal grafts under an IRB-approved protocol, and their phenotype was assessed by immunocytochemistry using a fluorophore-conjugated antibody against CK3/2p. The human amniotic membrane was isolated from the placenta and adhered to a genipin crosslinked collagen sheet/polycaprolactone (PCL) using the gluing solution. The morphology and composition of the surface layer were evaluated by scanning electron microscopy. PCL was laser cut using an Epilog laser system. The amnion section of bladder patch was seeded with human oral epithelial cells and cultured for three days following sterilization. Seeded collagen sheets were evaluated for proliferation using MTT assay. Western blot for CK3/2p was performed on cells seeded on bladder patch to determine their phenotype at 72 hours.
Results: Human epithelial cells stained positive for CK3/2p. SEM showed a connection between layers within composite bladder patch. Epithelial cells exhibited increased cell proliferation when seeded on bladder patch compared to epithelial cells that are seeded on the culture plate (p < 0.05). Protein expression highlighted the presence of CK3/2p at 72 hours following seeding on a bladder patch.
Conclusions: Multiphasic bladder patch supports sufficient epithelial cell attachment and survival suitable for implantation. Protein expression results suggest that a multiphasic bladder patch sustains the epithelial cell phenotype and increases the proliferation rate. The proposed bioengineered bladder patch is highly novel, can be utilized in conjunction with epithelial cells and has tremendous potential for regenerative medicine-based repair of bladder tissue.
Source of Funding: UH Cutler Fund
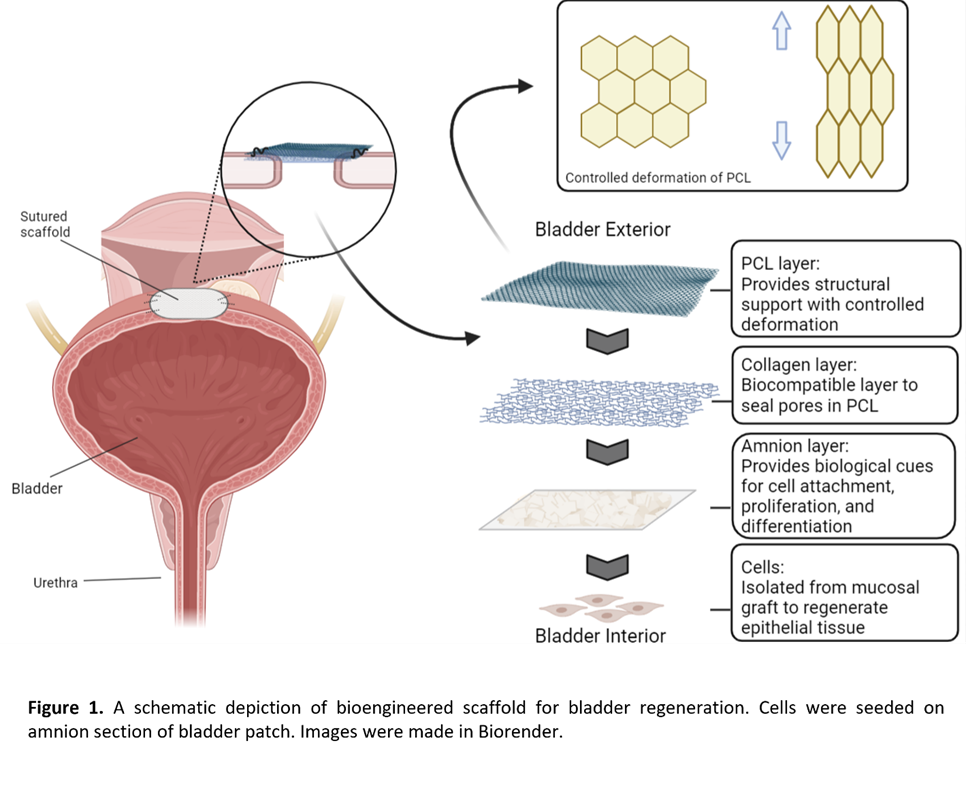
Methods: Human oral epithelial cells were harvested from buccal grafts under an IRB-approved protocol, and their phenotype was assessed by immunocytochemistry using a fluorophore-conjugated antibody against CK3/2p. The human amniotic membrane was isolated from the placenta and adhered to a genipin crosslinked collagen sheet/polycaprolactone (PCL) using the gluing solution. The morphology and composition of the surface layer were evaluated by scanning electron microscopy. PCL was laser cut using an Epilog laser system. The amnion section of bladder patch was seeded with human oral epithelial cells and cultured for three days following sterilization. Seeded collagen sheets were evaluated for proliferation using MTT assay. Western blot for CK3/2p was performed on cells seeded on bladder patch to determine their phenotype at 72 hours.
Results: Human epithelial cells stained positive for CK3/2p. SEM showed a connection between layers within composite bladder patch. Epithelial cells exhibited increased cell proliferation when seeded on bladder patch compared to epithelial cells that are seeded on the culture plate (p < 0.05). Protein expression highlighted the presence of CK3/2p at 72 hours following seeding on a bladder patch.
Conclusions: Multiphasic bladder patch supports sufficient epithelial cell attachment and survival suitable for implantation. Protein expression results suggest that a multiphasic bladder patch sustains the epithelial cell phenotype and increases the proliferation rate. The proposed bioengineered bladder patch is highly novel, can be utilized in conjunction with epithelial cells and has tremendous potential for regenerative medicine-based repair of bladder tissue.
Source of Funding: UH Cutler Fund


.jpg)
.jpg)