Back
Objectives: High levels of plasma LDL cholesterol are an important determinant of atherosclerotic lesion formation. The disruption of the process of cholesterol efflux or reverse cholesterol transport (RCT) of peripheral cells may promote atherogenesis. The aim of the current study was to investigate whether ellagic acid alleviated atherosclerotic development through activating RCT pathway in apoE knockout (KO) mice.
Methods: Wild type mice and apoE KO mice were fed high-fat high-cholesterol Paigen’s diets for 10 weeks to induce hypercholesterolemia and atherosclerosis, and concomitantly received 10 mg/kg ellagic acid via gavage.
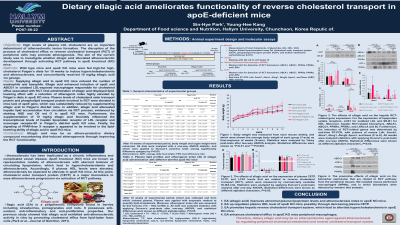
Results: Supplying ellagic acid to apoE KO mice reduced the number of eosinophils and basophils. Ellagic acid enhanced induction of apoE and ABCG1 in oxidized LDL-exposed macrophages responsible for cholesterol efflux associated with RCT. Oral administration of ellagic acid displayed lipid-lowering effect with a reduction of atherogenic index highly elevated by Paigen’s diets in apoE KO mice. Plasma levels of cholesterol ester transport protein and phospholipid transport protein involved in RCT were elevated in mice lack of apoE gene, which was substantially reduced by supplementing ellagic acid to Paigen’s diet-fed mice. In addition, ellagic acid attenuated hepatic lipid accumulation from circulation via RCT process, evidenced by staining of H&E and Oil red O in apoE KO mice. Furthermore, the supplementation of 10 mg/kg ellagic acid favorably influenced the transcriptional levels of hepatic lipoprotein receptor of LDL receptor and scavenger receptor-B1 in Paigen’s diet-fed apoE KO mice. Finally, the signaling of PPAR-liver X receptor α appeared to be involved in the lipid-lowering ability of ellagic acid in apoE KO mice.
Conclusions: Ellagic acid may be an athero-protective dietary compound encumbering diet-induced atherogenesis though improving the RCT functionality.
Funding Sources: This work was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2021R1A6A1A03044501).
Dietary Bioactive Components
(PO07-58-22) Dietary Ellagic Acid Ameliorates Functionality of Reverse Cholesterol Transport in apoE-Deficient Mice

- SP
Sin-Hye Park, PhD
– Research Professor, Hallym University, Chuncheon, Kangwon-do, Republic of Korea - YK
Young-Hee Kang, Ph.D
– Hallym University, chuncheon, Kangwon-do, Republic of Korea
Presenting Author(s)
Co-Author(s)
Disclosure(s):
Sin-Hye Park, PhD: No relevant financial relationship(s) with ineligible companies to disclose.
Objectives: High levels of plasma LDL cholesterol are an important determinant of atherosclerotic lesion formation. The disruption of the process of cholesterol efflux or reverse cholesterol transport (RCT) of peripheral cells may promote atherogenesis. The aim of the current study was to investigate whether ellagic acid alleviated atherosclerotic development through activating RCT pathway in apoE knockout (KO) mice.
Methods: Wild type mice and apoE KO mice were fed high-fat high-cholesterol Paigen’s diets for 10 weeks to induce hypercholesterolemia and atherosclerosis, and concomitantly received 10 mg/kg ellagic acid via gavage.
Results: Supplying ellagic acid to apoE KO mice reduced the number of eosinophils and basophils. Ellagic acid enhanced induction of apoE and ABCG1 in oxidized LDL-exposed macrophages responsible for cholesterol efflux associated with RCT. Oral administration of ellagic acid displayed lipid-lowering effect with a reduction of atherogenic index highly elevated by Paigen’s diets in apoE KO mice. Plasma levels of cholesterol ester transport protein and phospholipid transport protein involved in RCT were elevated in mice lack of apoE gene, which was substantially reduced by supplementing ellagic acid to Paigen’s diet-fed mice. In addition, ellagic acid attenuated hepatic lipid accumulation from circulation via RCT process, evidenced by staining of H&E and Oil red O in apoE KO mice. Furthermore, the supplementation of 10 mg/kg ellagic acid favorably influenced the transcriptional levels of hepatic lipoprotein receptor of LDL receptor and scavenger receptor-B1 in Paigen’s diet-fed apoE KO mice. Finally, the signaling of PPAR-liver X receptor α appeared to be involved in the lipid-lowering ability of ellagic acid in apoE KO mice.
Conclusions: Ellagic acid may be an athero-protective dietary compound encumbering diet-induced atherogenesis though improving the RCT functionality.
Funding Sources: This work was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2021R1A6A1A03044501).

.png)
