Back

Poster Session
(GB-07) The potential mechanism of CTLA-4-Ig-mediated osteoclast differentiation

Has Audio

Saki Nakane-Koyachi, PhD
Resident
Department of Periodontology, Tokyo Dental College
Lead Author(s)
Background and objective:
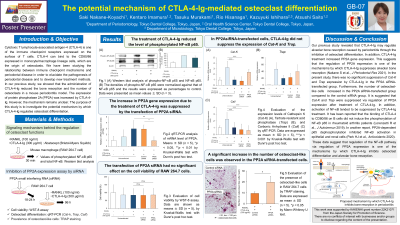
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) is one of the immune checkpoint receptors expressed on the surface of T cells. In our previous study, we showed that the administration of CTLA-4-Ig reduced the bone resorption and the number of osteoclasts in a mouse periodontitis model. The expression of protein phosphatase 2A (PP2A) was increased by CTLA-4-Ig. However, the mechanism remains unclear. The purpose of this study is to investigate the potential mechanism by which CTLA-4-Ig regulates osteoclast differentiation.
Materials and
Methods:
RANKL and CTLA-4-Ig were added to RAW264.7 cells. The effect of CTLA-4-Ig on nuclear factor kappa B (NF-κB) activation was evaluated by western blot analysis. Gene expression of PP2A in RAW264.7 cells was suppressed by siRNA transfection. Cell viability was evaluated by WST-8 assay. The expression of osteoclast differentiation markers (Cathepsin K, Trap) was assessed by qRT-PCR, and the number of osteoclast-like cells was evaluated by TRAP staining in transfected cells.
Results:
NF-κB phosphorylation was suppressed by the treatment of CTLA-4-Ig. The siRNA transfection had no significant effect on cell viability. In the PP2A siRNA transfected group, CTLA-4-Ig induced no significant suppression of Cathepsin K and Trap expressions. The number of osteoclast-like cells tended to increase in the PP2A siRNA transfected group compared to the control siRNA transfected group.
Conclusion:
These data suggested that regulation of the NF-κB pathway via modulation of PP2A expression is one of the mechanisms by which CTLA-4-Ig inhibits osteoclast differentiation.
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) is one of the immune checkpoint receptors expressed on the surface of T cells. In our previous study, we showed that the administration of CTLA-4-Ig reduced the bone resorption and the number of osteoclasts in a mouse periodontitis model. The expression of protein phosphatase 2A (PP2A) was increased by CTLA-4-Ig. However, the mechanism remains unclear. The purpose of this study is to investigate the potential mechanism by which CTLA-4-Ig regulates osteoclast differentiation.
Materials and
Methods:
RANKL and CTLA-4-Ig were added to RAW264.7 cells. The effect of CTLA-4-Ig on nuclear factor kappa B (NF-κB) activation was evaluated by western blot analysis. Gene expression of PP2A in RAW264.7 cells was suppressed by siRNA transfection. Cell viability was evaluated by WST-8 assay. The expression of osteoclast differentiation markers (Cathepsin K, Trap) was assessed by qRT-PCR, and the number of osteoclast-like cells was evaluated by TRAP staining in transfected cells.
Results:
NF-κB phosphorylation was suppressed by the treatment of CTLA-4-Ig. The siRNA transfection had no significant effect on cell viability. In the PP2A siRNA transfected group, CTLA-4-Ig induced no significant suppression of Cathepsin K and Trap expressions. The number of osteoclast-like cells tended to increase in the PP2A siRNA transfected group compared to the control siRNA transfected group.
Conclusion:
These data suggested that regulation of the NF-κB pathway via modulation of PP2A expression is one of the mechanisms by which CTLA-4-Ig inhibits osteoclast differentiation.
