Back
Poster Session C
Epidemiology, health policy and outcomes
Session: (1186–1214) Epidemiology and Public Health Poster II
1208: Development and Validation of a Prognostic Model for Methotrexate Discontinuation with Abnormal Monitoring Blood-test Results: A Cohort Study Using Data from Clinical Practice Research Datalink Gold and Aurum
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- GN
Georgina Nakafero, PhD
University of Nottingham
Nottingham, United Kingdom
Abstract Poster Presenter(s)
Georgina Nakafero1, Matthew J. Grainge1, Tim Card1, Maarten W Taal1, Guruprasad P Aithal1, Christopher P Fox2, Christian Mallen3, Danielle van der Windt3, Richard Riley3 and Abhishek Abhishek1, 1University of Nottingham, Nottingham, United Kingdom, 2Nottingham University Hospital NHS Trust, Nottingham, United Kingdom, 3Keele University, Keele, United Kingdom
Background/Purpose: Patients established on long-term low-dose weekly methotrexate treatment undergo 3-monthly monitoring blood-tests to facilitate early detection of drug-related adverse events. Regular testing may not be necessary for those at low risk whereas patients at high-risk may require more frequent monitoring or be prescribed alternate drugs. Thus, the aim of this study was to develop and externally validate a prognostic model for methotrexate discontinuation with abnormal blood-test results that can be used to inform clinical practice.
Methods: Data from Clinical Practice Research Datalink (CPRD) Gold and Aurum were used for model development and external validation respectively. CPRD Gold and Aurum have complementary coverage of GP practices, and this allowed for true external validation. Patients with immune-mediated inflammatory diseases such as rheumatoid arthritis, psoriasis +/- arthritis, connective tissue disease etc. prescribed methotrexate between 01/01/2007 and 31/12/2019 were followed-up from six-months after their first shared-care GP-prescription to the earliest of outcome date, death date, 5-year follow-up or 31/12/2019. Multiple imputation was applied to handle missing data. All candidate predictors were included in the Cox model. The equation for predicting an individual's risk of methotrexate discontinuation at 5-years was formulated. Internal validation using 500-bootstrap samples was undertaken to correct for optimism. Regression coefficients were penalised using the uniform shrinkage factor, estimated as the average of calibration slopes from each of the bootstrap samples. Model performance was assessed in terms of calibration and discrimination.
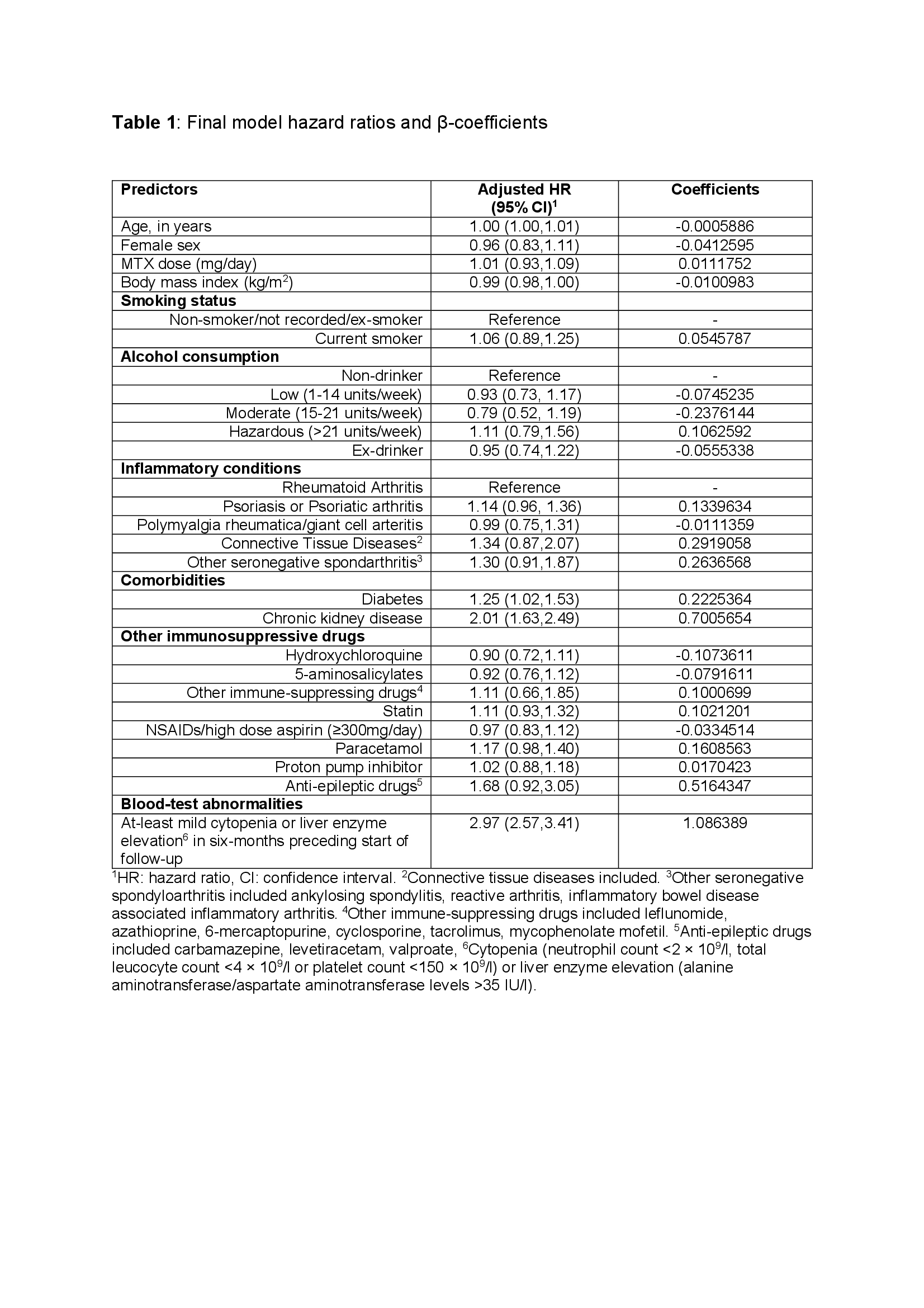
Results: Data for 13,110 and 23,999 participants were included in the development and validation cohorts, respectively. Majority of participants in the development and validation cohort were female; 63.1% vs. 63.6%, had rheumatoid arthritis; 61.8% vs.62.8% and had comparable prevalence of adverse lifestyle factors, comorbidities, and prescriptions. Diabetes, chronic kidney disease and mild cytopenia or elevated liver enzymes during the six-month of methotrexate prescription prior to start of follow-up were strong predictors of drug discontinuation with adjusted hazard ratio (95% confidence interval (CI)) 1.25 (1.02-1.53), 2.01 (1.63-2.49) and 2.97 (2.57-3.41) respectively (Table 1).
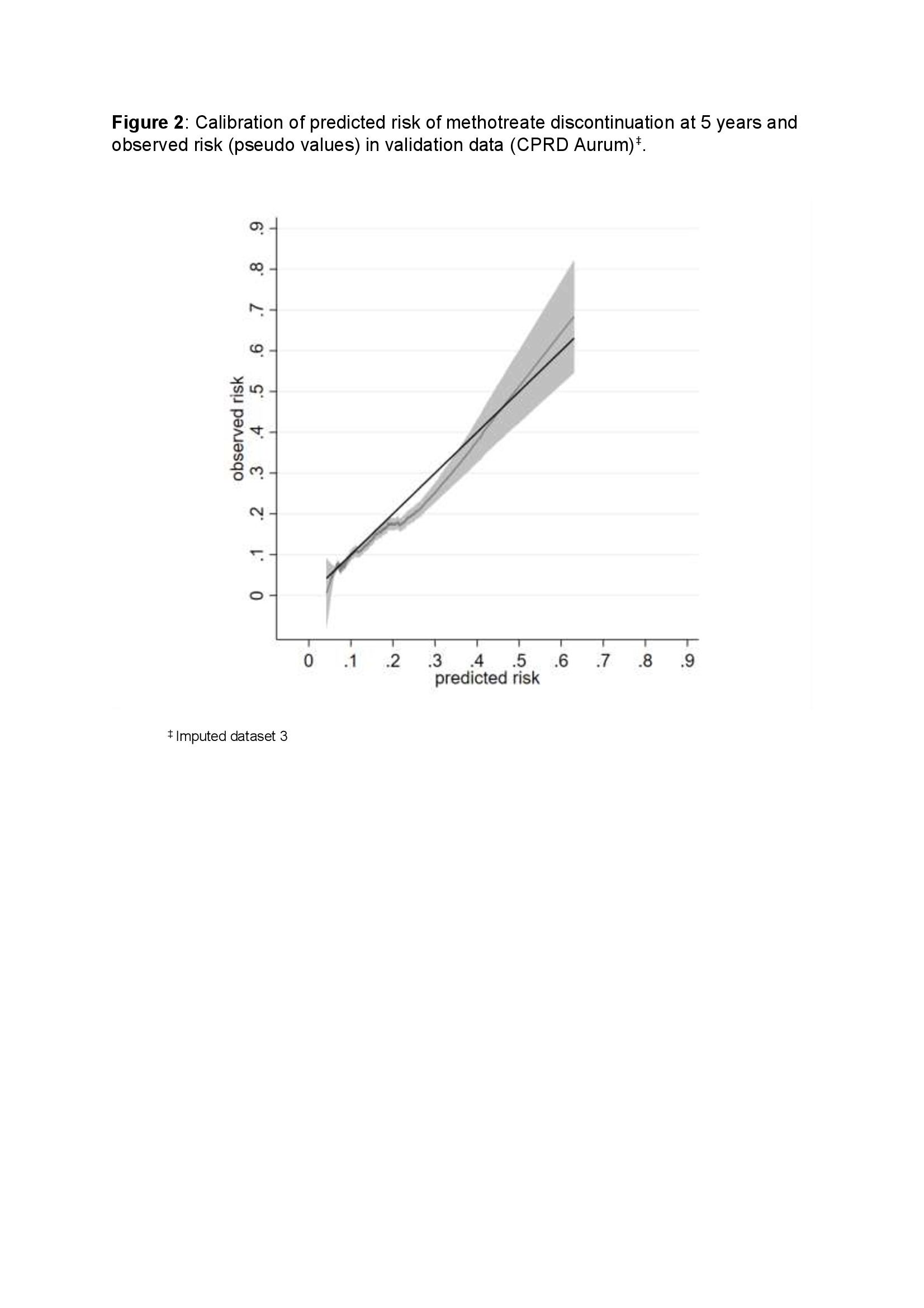
The baseline survival function was 0.895 and uniform shrinkage factor was 0.93 used to calculate methotrexate discontinuation risk score (Figure 1). The optimism unadjusted and adjusted calibration slope (95% CI) in development data was 1.00 (0.89-1.11) and 0.93 (0.82- 1.04) respectively. The calibration slope (95% CI) in validation data was 0.94 (0.85- 1.02) (Figure 2). The model showed prognostic separation with Royston D statistic (95%CI) of 0.79 (0.69-0.90) and 0.75 (0.67-0.83) in the development and external validation data respectively.
Conclusion: This prognostic model used predictors available during routine clinical consultations and should be used to risk-stratify blood-test monitoring after the first year of methotrexate prescription.

Disclosures: G. Nakafero, None; M. Grainge, None; T. Card, None; M. Taal, None; G. Aithal, AstraZeneca; C. Fox, None; C. Mallen, None; D. van der Windt, None; R. Riley, None; A. Abhishek, AstraZeneca, Pfizer, Oxford immunotec, Inflazome, NGMBiopharmaceuticals, Uptodate, Cadilla pharmaceuticals.
Background/Purpose: Patients established on long-term low-dose weekly methotrexate treatment undergo 3-monthly monitoring blood-tests to facilitate early detection of drug-related adverse events. Regular testing may not be necessary for those at low risk whereas patients at high-risk may require more frequent monitoring or be prescribed alternate drugs. Thus, the aim of this study was to develop and externally validate a prognostic model for methotrexate discontinuation with abnormal blood-test results that can be used to inform clinical practice.
Methods: Data from Clinical Practice Research Datalink (CPRD) Gold and Aurum were used for model development and external validation respectively. CPRD Gold and Aurum have complementary coverage of GP practices, and this allowed for true external validation. Patients with immune-mediated inflammatory diseases such as rheumatoid arthritis, psoriasis +/- arthritis, connective tissue disease etc. prescribed methotrexate between 01/01/2007 and 31/12/2019 were followed-up from six-months after their first shared-care GP-prescription to the earliest of outcome date, death date, 5-year follow-up or 31/12/2019. Multiple imputation was applied to handle missing data. All candidate predictors were included in the Cox model. The equation for predicting an individual's risk of methotrexate discontinuation at 5-years was formulated. Internal validation using 500-bootstrap samples was undertaken to correct for optimism. Regression coefficients were penalised using the uniform shrinkage factor, estimated as the average of calibration slopes from each of the bootstrap samples. Model performance was assessed in terms of calibration and discrimination.
Results: Data for 13,110 and 23,999 participants were included in the development and validation cohorts, respectively. Majority of participants in the development and validation cohort were female; 63.1% vs. 63.6%, had rheumatoid arthritis; 61.8% vs.62.8% and had comparable prevalence of adverse lifestyle factors, comorbidities, and prescriptions. Diabetes, chronic kidney disease and mild cytopenia or elevated liver enzymes during the six-month of methotrexate prescription prior to start of follow-up were strong predictors of drug discontinuation with adjusted hazard ratio (95% confidence interval (CI)) 1.25 (1.02-1.53), 2.01 (1.63-2.49) and 2.97 (2.57-3.41) respectively (Table 1).
The baseline survival function was 0.895 and uniform shrinkage factor was 0.93 used to calculate methotrexate discontinuation risk score (Figure 1). The optimism unadjusted and adjusted calibration slope (95% CI) in development data was 1.00 (0.89-1.11) and 0.93 (0.82- 1.04) respectively. The calibration slope (95% CI) in validation data was 0.94 (0.85- 1.02) (Figure 2). The model showed prognostic separation with Royston D statistic (95%CI) of 0.79 (0.69-0.90) and 0.75 (0.67-0.83) in the development and external validation data respectively.
Conclusion: This prognostic model used predictors available during routine clinical consultations and should be used to risk-stratify blood-test monitoring after the first year of methotrexate prescription.



Disclosures: G. Nakafero, None; M. Grainge, None; T. Card, None; M. Taal, None; G. Aithal, AstraZeneca; C. Fox, None; C. Mallen, None; D. van der Windt, None; R. Riley, None; A. Abhishek, AstraZeneca, Pfizer, Oxford immunotec, Inflazome, NGMBiopharmaceuticals, Uptodate, Cadilla pharmaceuticals.

