Back
Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1486–1517) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster III
1496: Do Patients in MDA Report Low Disease Activity Regardless of Which of the MDA Criteria Are Met?
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- SY
Sarah Yazji, BSc, MBBCH
Oxford University NHS Foundation Trust
Oxford, United Kingdom
Abstract Poster Presenter(s)
Sarah Yazji1, Philip Helliwell2, Andra Balanescu3, JUAN CANETE4, Emmanuelle Dernis5, Uta Kiltz6, Ying Ying Leung7, Ennio Lubrano8, Ana-Maria Orbai9, PENELOPE PALOMINOS10, Rossana Scrivo11, Josef Smolen12, Sandra Meisalu13, Maarten de Wit14, Laure Gossec15 and Laura Coates16, 1University of Oxford, Oxford, United Kingdom, 2Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, United Kingdom, 3University of Medicine and Pharmacy Carol Davila, Bucharest, Romania, 4Rheumatology Department, Hospital Clinic and IDIBAPS, Barcelona, Spain, 5LE MANS general hospital, LE MANS, France, 6Rheumazentrum Ruhrgebiet, Herne, Germany, 7Rheumatology Department, Singapore General Hospital, Singapore, Singapore, 8Academic Rheumatology Unit, Dipartimento di Medicina e Scienze della Salute ‘‘Vincenzo Tiberio’’, Università degli Studi del Molise, Campobasso, Italy, 9Johns Hopkins University School of Medicine, Division of Rheumatology, Baltimore, MD, 10Hospital de Clinicas de Porto Alegre, Porto Alegre, Rio Grande do Sul, Brazil, 11Rheumatology Unit, Department of Clinical Internal, Anesthesiological and Cardiovascular Sciences, Sapienza University of Rome, Rome, Italy, Roma, Rome, Italy, 12Medical University of Vienna, Vienna, Austria, 13East Tallinn Central Hospital, Tallinn, Estonia, 14Patient research partner, Amsterdam, Netherlands, 15Sorbonne Université, Paris, France, 16Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK, Oxford, England, United Kingdom
Background/Purpose: The Minimal Disease Activity (MDA) criteria evaluate PsA disease activity and response to treatment and are defined as meeting ≥5 criteria: tender joint count ≤1, swollen joint count ≤1, patient global visual analogue scale (PG VAS) ≤20mm, pain VAS ≤15mm, health assessment questionnaire (HAQ) ≤0.5, enthesitis count ≤1 and psoriasis body surface area ≤3%.
The EMA recently published a qualification letter of support for MDA but asked: Is there evidence showing 'minimal disease activity' as measured by the MDA score is also perceived as such by the patient regardless of which of the 5 criteria are met?
Methods: We analysed data from patients in ReFlap (NCT03119805), a cross-sectional international study of adult patients with PsA for >2 years. Patients self-reported remission (REM) or low disease activity (LDA) by answering positively to the questions 'At this time, is your psoriatic arthritis in remission, if that means you feel your disease is as good as gone?' and 'At this time, are you in low disease activity, if this means your disease is in low activity but it's not as good as gone?' respectively.
For patients in MDA the frequencies of criteria met were examined. Odds ratios examined whether there is an increase in the odds of a patient not reporting LDA/REM when a criterion is not met. Chi squared tests, Fisher's exact and Yates' correction were used to compare patient-reported LDA/REM against MDA with different individual criteria. Specificity and positive predictive value (PPV) of MDA were calculated for when each criterion was not met to identify if patients are being over-detected as having low disease activity by the MDA score.
Results: Of the 466 recruited participants, 420 had complete data. Of the 420, 159 patients (37.9%) met MDA and 105 patients (25%) met MDA but not VLDA (i.e. 5-6 criteria). 15 of the patients who met MDA criteria did not self-report LDA/REM (Fig A).
The most common criteria not met within MDA were pain VAS (not met in 42.1% of MDA patients) and PG VAS (not met in 30%.) Odds ratios showed no significant difference in the odds of a patient self-reporting LDA/REM when a criterion is not met (Table 2). The chi square analyses also showed no significant difference between criteria met, however group sizes were small. Fisher's exact test, more accurate in smaller sample sizes, confirmed the chi squared findings (Table 1).
Specificity was consistently high ( >90%) for MDA score overall and MDA with each criteria missing suggesting the tool did not over-detect patients to be in MDA no matter which criterion was not individually met (Table 2).
The same analyses were performed for when both PG VAS and pain VAS were not met as this was commonest when only 5 criteria were met. No significant differences were found. Specificity remained high (95.2%).
Conclusion: The likelihood of a patient self-reporting LDA/REM and specificity of MDA score were unaffected by which MDA criteria were met. Pain VAS and PG VAS were the hardest criteria to meet in this analysis, but not meeting them was independent of patients reporting LDA/REM. High specificity and PPV identified for MDA are especially relevant to clinical practice where it is important to reliably detect patients with active disease.
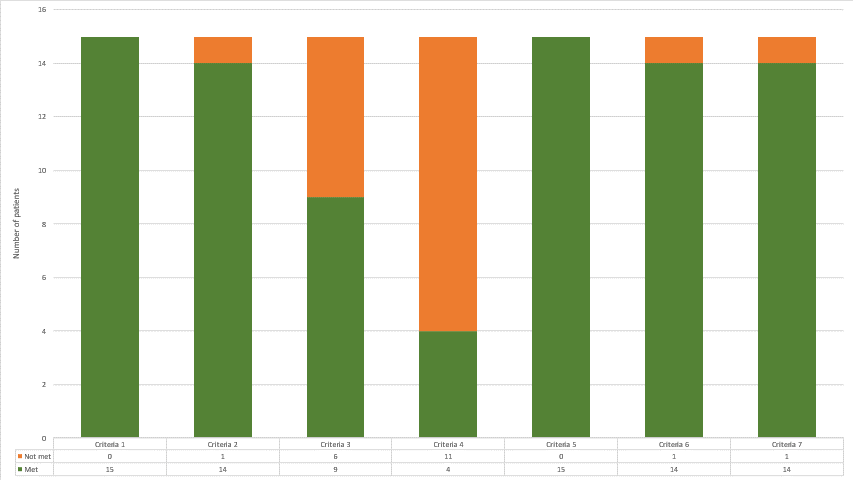 Figure 1: Frequencies of criteria met/not met for patients in MDA who did not self-report low disease activity/remission (n=15). TJC=tender joint count, SJC=swollen joint count, HAQ=health assessment questionnaire, BSA=body surface area
Figure 1: Frequencies of criteria met/not met for patients in MDA who did not self-report low disease activity/remission (n=15). TJC=tender joint count, SJC=swollen joint count, HAQ=health assessment questionnaire, BSA=body surface area
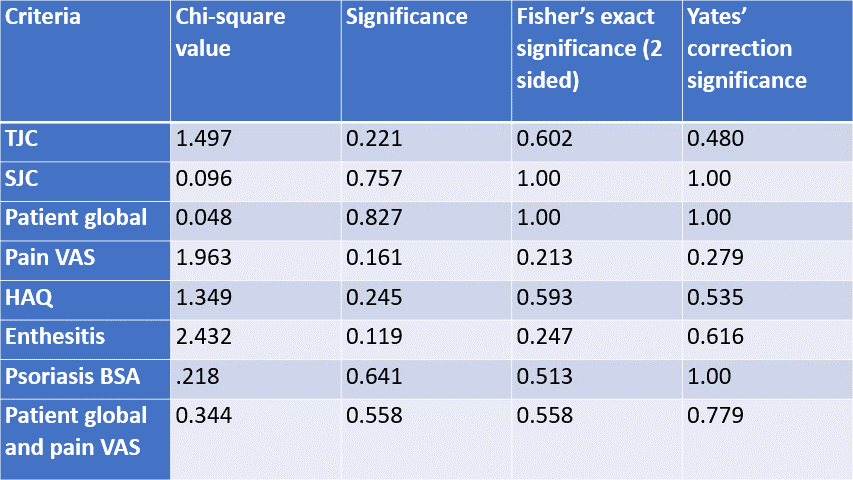 Table 1: Chi square, Fisher's exact p value and Yates' correction p value comparing self-reported low disease activity/remission to meeting each criterion for patients who met minimal disease activity (MDA) but not very low disease activity (VLDA)
Table 1: Chi square, Fisher's exact p value and Yates' correction p value comparing self-reported low disease activity/remission to meeting each criterion for patients who met minimal disease activity (MDA) but not very low disease activity (VLDA)
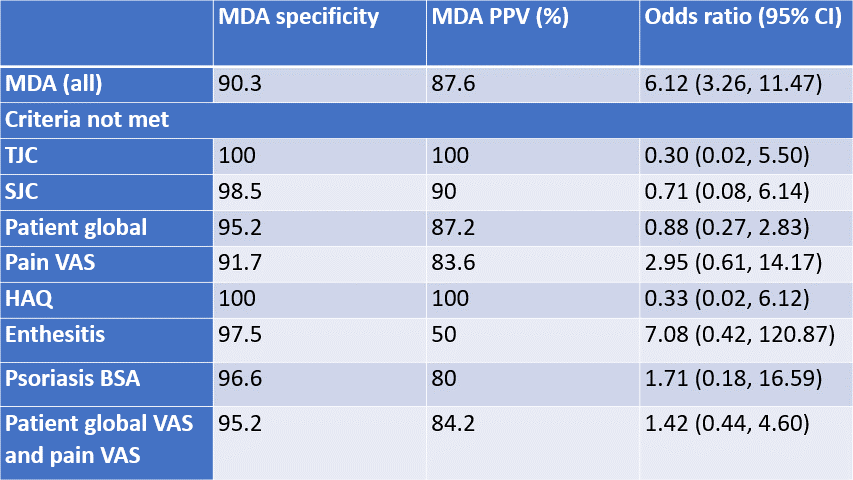 Table 2: Specificity analysis and PPV for MDA criteria and for when each criterion is not met. Odds ratios are the odds of a patient self-reporting LDA/REM when meeting MDA in total, and if each individual criteria is missed. PPV=positive predictive value, CI=confidence interval
Table 2: Specificity analysis and PPV for MDA criteria and for when each criterion is not met. Odds ratios are the odds of a patient self-reporting LDA/REM when meeting MDA in total, and if each individual criteria is missed. PPV=positive predictive value, CI=confidence interval
Disclosures: S. Yazji, None; P. Helliwell, Eli Lilly, AbbVie, Amgen, Janssen, Novartis; A. Balanescu, AbbVie; J. CANETE, None; E. Dernis, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Janssen, Nordic Pharma France, Novartis, UCB; U. Kiltz, AbbVie, Amgen, Biogen, Fresenius, GSK, Hexal, Novartis, Pfizer, Biocad, Lilly, Grünenthal, Janssen, MSD, Roche, UCB; Y. Leung, AbbVie/Abbott, Janssen, Pfizer, DKSH; E. Lubrano, None; A. Orbai, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Janssen, UCB; P. PALOMINOS, None; R. Scrivo, None; J. Smolen, AbbVie, AstraZeneca, Eli Lilly, Novartis, Amgen, Bristol Myers Squibb, Galapagos-Gilead, Janssen, Merck-Sharp-Dohme, Novartis-Sandoz, Pfizer, Roche-Chugai, Samsung, UCB; S. Meisalu, None; M. de Wit, Stiching Tools, Celgene, Eli Lilly, Pfizer, UCB; L. Gossec, Amgen, Lilly, Pfizer, Sandoz, UCB Pharma, AbbVie, Bristol Myers Squibb, Gilead, Janssen, Novartis, Samsung Bioepis, Sanofi-Aventis, Galapagos, GlaxoSmithKlein (GSK), Celltrion, MSD; L. Coates, AbbVie, Amgen, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, Gilead, Galapagos, Janssen, Medac, Novartis, Pfizer, UCB, Celgene, Biogen, Moonlake, GlaxoSmithKlein (GSK).
Background/Purpose: The Minimal Disease Activity (MDA) criteria evaluate PsA disease activity and response to treatment and are defined as meeting ≥5 criteria: tender joint count ≤1, swollen joint count ≤1, patient global visual analogue scale (PG VAS) ≤20mm, pain VAS ≤15mm, health assessment questionnaire (HAQ) ≤0.5, enthesitis count ≤1 and psoriasis body surface area ≤3%.
The EMA recently published a qualification letter of support for MDA but asked: Is there evidence showing 'minimal disease activity' as measured by the MDA score is also perceived as such by the patient regardless of which of the 5 criteria are met?
Methods: We analysed data from patients in ReFlap (NCT03119805), a cross-sectional international study of adult patients with PsA for >2 years. Patients self-reported remission (REM) or low disease activity (LDA) by answering positively to the questions 'At this time, is your psoriatic arthritis in remission, if that means you feel your disease is as good as gone?' and 'At this time, are you in low disease activity, if this means your disease is in low activity but it's not as good as gone?' respectively.
For patients in MDA the frequencies of criteria met were examined. Odds ratios examined whether there is an increase in the odds of a patient not reporting LDA/REM when a criterion is not met. Chi squared tests, Fisher's exact and Yates' correction were used to compare patient-reported LDA/REM against MDA with different individual criteria. Specificity and positive predictive value (PPV) of MDA were calculated for when each criterion was not met to identify if patients are being over-detected as having low disease activity by the MDA score.
Results: Of the 466 recruited participants, 420 had complete data. Of the 420, 159 patients (37.9%) met MDA and 105 patients (25%) met MDA but not VLDA (i.e. 5-6 criteria). 15 of the patients who met MDA criteria did not self-report LDA/REM (Fig A).
The most common criteria not met within MDA were pain VAS (not met in 42.1% of MDA patients) and PG VAS (not met in 30%.) Odds ratios showed no significant difference in the odds of a patient self-reporting LDA/REM when a criterion is not met (Table 2). The chi square analyses also showed no significant difference between criteria met, however group sizes were small. Fisher's exact test, more accurate in smaller sample sizes, confirmed the chi squared findings (Table 1).
Specificity was consistently high ( >90%) for MDA score overall and MDA with each criteria missing suggesting the tool did not over-detect patients to be in MDA no matter which criterion was not individually met (Table 2).
The same analyses were performed for when both PG VAS and pain VAS were not met as this was commonest when only 5 criteria were met. No significant differences were found. Specificity remained high (95.2%).
Conclusion: The likelihood of a patient self-reporting LDA/REM and specificity of MDA score were unaffected by which MDA criteria were met. Pain VAS and PG VAS were the hardest criteria to meet in this analysis, but not meeting them was independent of patients reporting LDA/REM. High specificity and PPV identified for MDA are especially relevant to clinical practice where it is important to reliably detect patients with active disease.
 Figure 1: Frequencies of criteria met/not met for patients in MDA who did not self-report low disease activity/remission (n=15). TJC=tender joint count, SJC=swollen joint count, HAQ=health assessment questionnaire, BSA=body surface area
Figure 1: Frequencies of criteria met/not met for patients in MDA who did not self-report low disease activity/remission (n=15). TJC=tender joint count, SJC=swollen joint count, HAQ=health assessment questionnaire, BSA=body surface area Table 1: Chi square, Fisher's exact p value and Yates' correction p value comparing self-reported low disease activity/remission to meeting each criterion for patients who met minimal disease activity (MDA) but not very low disease activity (VLDA)
Table 1: Chi square, Fisher's exact p value and Yates' correction p value comparing self-reported low disease activity/remission to meeting each criterion for patients who met minimal disease activity (MDA) but not very low disease activity (VLDA) Table 2: Specificity analysis and PPV for MDA criteria and for when each criterion is not met. Odds ratios are the odds of a patient self-reporting LDA/REM when meeting MDA in total, and if each individual criteria is missed. PPV=positive predictive value, CI=confidence interval
Table 2: Specificity analysis and PPV for MDA criteria and for when each criterion is not met. Odds ratios are the odds of a patient self-reporting LDA/REM when meeting MDA in total, and if each individual criteria is missed. PPV=positive predictive value, CI=confidence intervalDisclosures: S. Yazji, None; P. Helliwell, Eli Lilly, AbbVie, Amgen, Janssen, Novartis; A. Balanescu, AbbVie; J. CANETE, None; E. Dernis, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Janssen, Nordic Pharma France, Novartis, UCB; U. Kiltz, AbbVie, Amgen, Biogen, Fresenius, GSK, Hexal, Novartis, Pfizer, Biocad, Lilly, Grünenthal, Janssen, MSD, Roche, UCB; Y. Leung, AbbVie/Abbott, Janssen, Pfizer, DKSH; E. Lubrano, None; A. Orbai, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Janssen, UCB; P. PALOMINOS, None; R. Scrivo, None; J. Smolen, AbbVie, AstraZeneca, Eli Lilly, Novartis, Amgen, Bristol Myers Squibb, Galapagos-Gilead, Janssen, Merck-Sharp-Dohme, Novartis-Sandoz, Pfizer, Roche-Chugai, Samsung, UCB; S. Meisalu, None; M. de Wit, Stiching Tools, Celgene, Eli Lilly, Pfizer, UCB; L. Gossec, Amgen, Lilly, Pfizer, Sandoz, UCB Pharma, AbbVie, Bristol Myers Squibb, Gilead, Janssen, Novartis, Samsung Bioepis, Sanofi-Aventis, Galapagos, GlaxoSmithKlein (GSK), Celltrion, MSD; L. Coates, AbbVie, Amgen, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, Gilead, Galapagos, Janssen, Medac, Novartis, Pfizer, UCB, Celgene, Biogen, Moonlake, GlaxoSmithKlein (GSK).

