Back
Poster Session C
Pediatric autoimmune diseases: Kawasaki disease, juvenile dermatomyositis and juvenile localized scleroderma
Session: (1360–1386) Pediatric Rheumatology – Clinical Poster II: Connective Tissue Disease
1376: Eculizumab Is Safe as an Adjunctive Therapy in Systemic Lupus Erythematosus with Severe Refractory Nephritis with or Without Thrombotic Microangiopathy in Children
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- MP
Maria Pereira, MD
Baylor College of Medicine
Houston, TX, United States
Abstract Poster Presenter(s)
Maria Pereira1, Angela Chun1, Leigh Stubbs1 and Marietta De Guzman2, 1Baylor College of Medicine, Houston, TX, 2Baylor College of Medicine/ Texas Children's Hospital, Houston, TX
Background/Purpose: Eculizumab is a monoclonal antibody that prevents the cleavage of C5, which inhibits the formation of the terminal complement complex. It is approved in pediatric patients with atypical hemolytic uremic syndrome to inhibit complement mediated Thrombotic Microangiopathy (TMA), and its use in Systemic Lupus Erythematosus (SLE) is gaining popularity. The efficacy and safety of eculizumab in childhood-onset SLE is yet to be defined.
Methods: With approval for our institutional review board, we studied a single-center retrospective observational cohort of children diagnosed with SLE who were prescribed eculizumab for severe refractory lupus nephritis with or without TMA from 2014-2022. TMA was determined either histologically or clinically based on the combination of microangiopathic hemolytic anemia, thrombocytopenia, and renal impairment. Clinical, laboratory measures, indications for eculizumab, treatment regimens, outcomes, and adverse effects were described. A favorable outcome was defined as resolution of hematologic abnormalities and recovery of renal function based on age-defined lab parameters and clinical need for hemodialysis.
Results: Six patients with SLE received eculizumab as treatment for severe disease (Table 1). One patient with scleroderma overlap and multiorgan involvement was excluded. The majority of the patients (67%) exhibited a favorable response to eculizumab. At 4 weeks, 83% had full recovery in hemolysis and thrombocytopenia. Twopatients remained dialysis dependent, and of these, one patient had relapse requiring re-treatment in the setting of factor H antibodies, and the other patient had a history of medication non-compliance. One patient developed varicella zoster with no cutaneous or visceral organ dissemination. She improved with intravenous acyclovir and transitioned to oral valacyclovir for a total 14-day course. Another patient had recurrent infections including line infections (Pseudomonas aeruginosa and Candida albicans), antibiotic-associated Clostridioides difficile diarrhea, and Cytomegalovirus (CMV) viremia requiring ganciclovir as well as CMV immune globulin. However, no infections related to meningococcal disease or other encapsulated organisms were reported. No infusion related reactions were noted.
Conclusion: Based on this observation cohort, we recommend considering eculizumab as an adjunctive treatment for pediatric SLE patients with TMA. Since these patients are on multiple immunosuppressive agents, it is important to monitor for infectious complications. Additional studies are needed to determine the optimal treatment duration and the risk factors for future TMA events after discontinuation of eculizumab in this population.
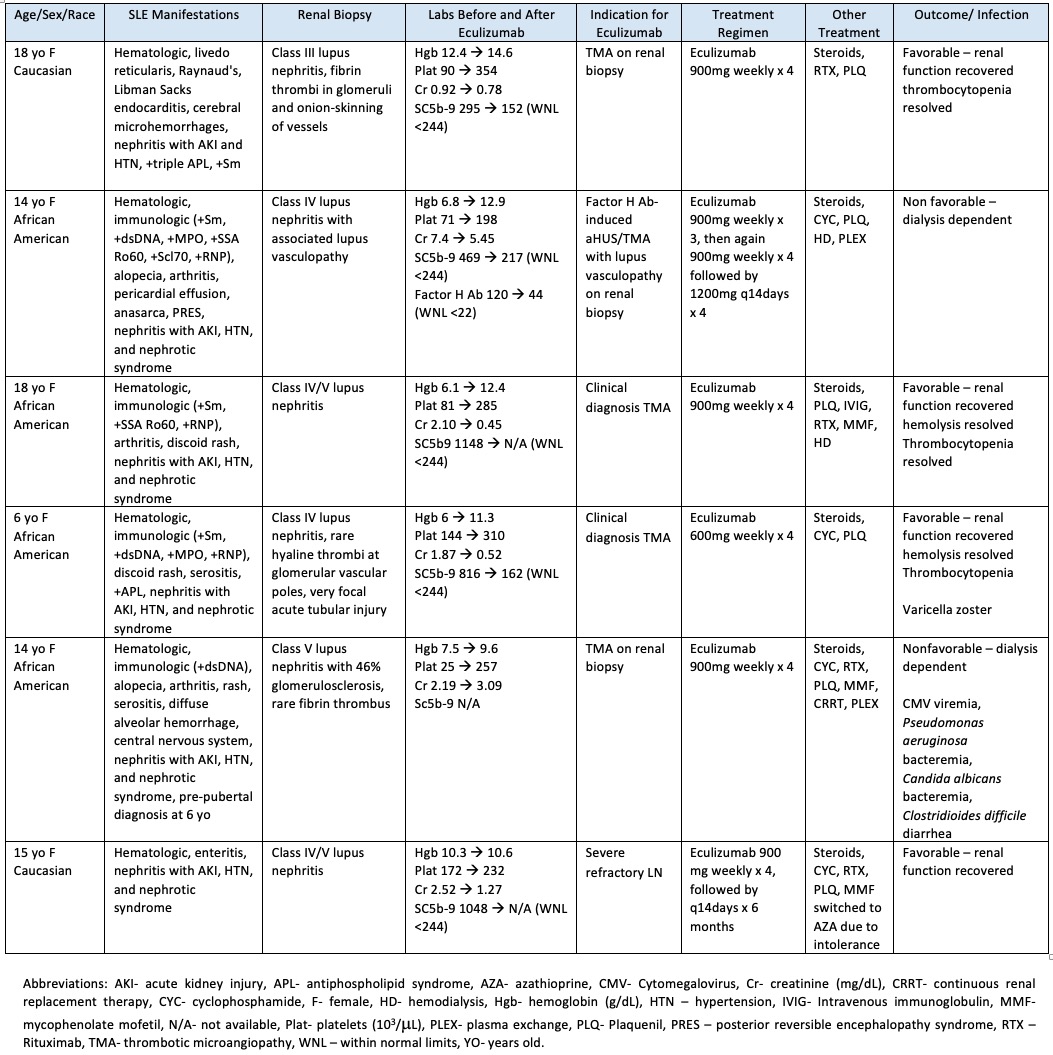 Table 1. Patient Cohort Characteristics
Table 1. Patient Cohort Characteristics
Disclosures: M. Pereira, None; A. Chun, None; L. Stubbs, None; M. De Guzman, None.
Background/Purpose: Eculizumab is a monoclonal antibody that prevents the cleavage of C5, which inhibits the formation of the terminal complement complex. It is approved in pediatric patients with atypical hemolytic uremic syndrome to inhibit complement mediated Thrombotic Microangiopathy (TMA), and its use in Systemic Lupus Erythematosus (SLE) is gaining popularity. The efficacy and safety of eculizumab in childhood-onset SLE is yet to be defined.
Methods: With approval for our institutional review board, we studied a single-center retrospective observational cohort of children diagnosed with SLE who were prescribed eculizumab for severe refractory lupus nephritis with or without TMA from 2014-2022. TMA was determined either histologically or clinically based on the combination of microangiopathic hemolytic anemia, thrombocytopenia, and renal impairment. Clinical, laboratory measures, indications for eculizumab, treatment regimens, outcomes, and adverse effects were described. A favorable outcome was defined as resolution of hematologic abnormalities and recovery of renal function based on age-defined lab parameters and clinical need for hemodialysis.
Results: Six patients with SLE received eculizumab as treatment for severe disease (Table 1). One patient with scleroderma overlap and multiorgan involvement was excluded. The majority of the patients (67%) exhibited a favorable response to eculizumab. At 4 weeks, 83% had full recovery in hemolysis and thrombocytopenia. Twopatients remained dialysis dependent, and of these, one patient had relapse requiring re-treatment in the setting of factor H antibodies, and the other patient had a history of medication non-compliance. One patient developed varicella zoster with no cutaneous or visceral organ dissemination. She improved with intravenous acyclovir and transitioned to oral valacyclovir for a total 14-day course. Another patient had recurrent infections including line infections (Pseudomonas aeruginosa and Candida albicans), antibiotic-associated Clostridioides difficile diarrhea, and Cytomegalovirus (CMV) viremia requiring ganciclovir as well as CMV immune globulin. However, no infections related to meningococcal disease or other encapsulated organisms were reported. No infusion related reactions were noted.
Conclusion: Based on this observation cohort, we recommend considering eculizumab as an adjunctive treatment for pediatric SLE patients with TMA. Since these patients are on multiple immunosuppressive agents, it is important to monitor for infectious complications. Additional studies are needed to determine the optimal treatment duration and the risk factors for future TMA events after discontinuation of eculizumab in this population.
 Table 1. Patient Cohort Characteristics
Table 1. Patient Cohort CharacteristicsDisclosures: M. Pereira, None; A. Chun, None; L. Stubbs, None; M. De Guzman, None.

