Back
Poster Session C
Pediatric autoimmune diseases: Kawasaki disease, juvenile dermatomyositis and juvenile localized scleroderma
Session: (1360–1386) Pediatric Rheumatology – Clinical Poster II: Connective Tissue Disease
1361: Factors Associated with Medication-Free Remission in Juvenile Dermatomyositis
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- HG
Harneet Ghumman, CCRC
Cincinnati Children's Hospital
Cincinnati, OH, United States
Abstract Poster Presenter(s)
Harneet Ghumman1, Asra Firdous1, Megan Quinlan-Waters1, Amy Cassedy2, Angela Merritt1, Hermine Brunner3, Alexei Grom1, Daniel Lovell1 and Sheila Angeles-Han1, 1Cincinnati Children's Hospital Medical Center, Cincinnati, OH, 2Cincinnati Children's Hospital Medical Center, Cleveland, OH, 3Division of Rheumatology, Cincinnati Children's Hospital Medical Center, University of Cincinnati, Department of Pediatrics, Cincinnati, OH
Background/Purpose: Juvenile dermatomyositis (JDM) is characterized by symmetric proximal muscle weakness, distinct rash, and a risk for calcinosis and multi-organ involvement. Treatment with systemic immunosuppression is needed. Evidence is limited on the factors associated with disease remission off of medication.
The aims of this study are to describe the demographic, clinical, laboratory, imaging and histological factors in our JDM cohort, and to identify variables associated with medication-free remission.
Methods: This is a retrospective study of a convenience sample of children diagnosed with JDM at ≤18 years of age by a pediatric rheumatologist, with a minimum clinical follow-up of 2 years. Medication-free remission was defined as inactive JDM off all systemic medications for at least 6 months. Medical records were reviewed. We compared the following variables in children who achieved remission to those who did not: demographics, clinical features, muscle enzymes, ANA, myositis-specific auto-antibodies, Childhood Myositis Assessment Scale (CMAS; to assess disease activity), Physician Global Assessment Scores, and Parent/Patient Global Assessment Scores. Group differences were tested using Fisher's Exact tests and Wilcoxon Rank Sum tests. A p< 0.05 was considered statistically significant. Analysis was conducted using SAS 9.3©.
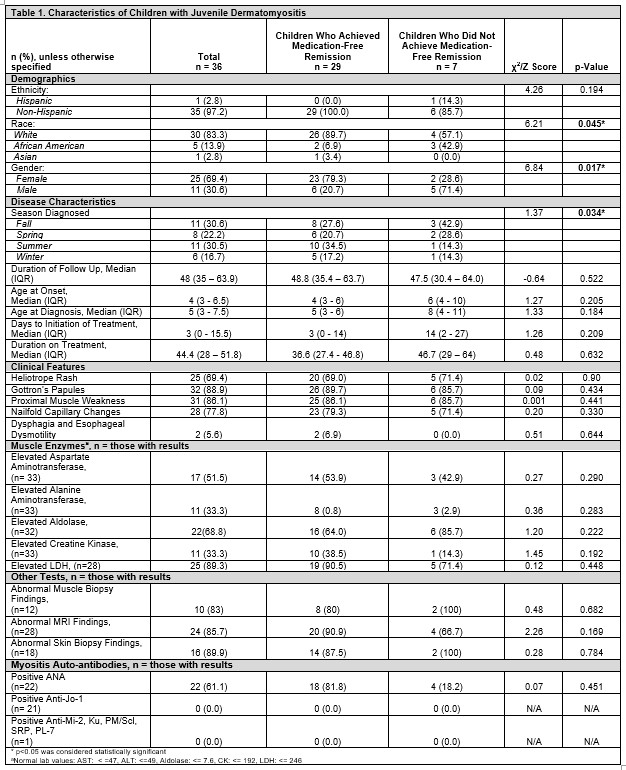
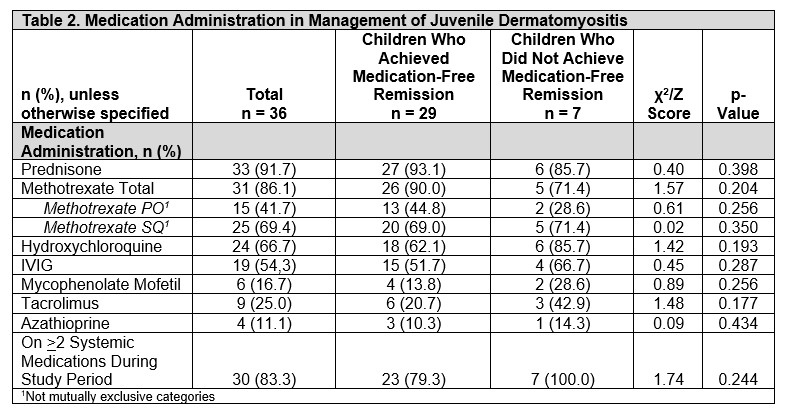
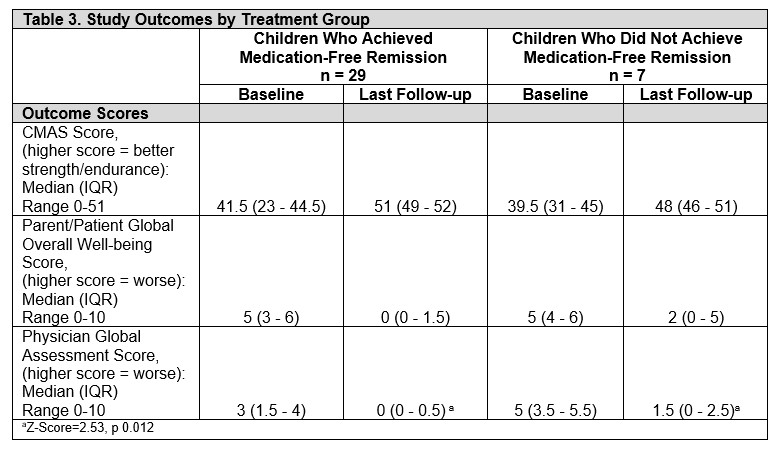
Results: Of 36 participants, 29 (80.5%) achieved medication-free remission while 7 (19.4%) were still on medication at last follow-up. Overall, most were non-Hispanic White (83%), females (69%), had a median age of JDM onset 4 years (range 3-6.5), and diagnosed in the fall (11, 30.6%) and summer (11, 30.5%). Those who achieved remission required treatment for 37 (interquartile [IQR] 27-47) months before discontinuation. Comparing children who achieved medication-free remission to those who did not, median age at JDM onset (4 [IQR 3-6] vs 6 [4-10] years), JDM diagnosis (5 [3-6] v. 8 [4-11] years), and time to treatment initiation (3 [0-14] vs 14 [2-27] days) was not statistically significant. However, more males (71% [n=5] vs. 21% [6], p=0.017) and African Americans (43% [3] vs. 7% [2], p=0.045) did not achieve remission. Further, most of these patients were diagnosed in the fall (43% [3] vs. 28% [8], p=0.034). Both groups had similar presentation of rash, muscle weakness, elevated muscle enzyme levels, ANA positivity, abnormal muscle and skin biopsy, and MRI findings (in those who had them done). 92% were treated with glucocorticoids. Methotrexate (86%) was the most commonly prescribed DMARD. There were no differences in systemic medications given over the disease course between both groups (Table 2). At last follow up, children who did not achieve remission had worse physician global assessment scores (0 [0-0.5] vs 1.5 [0-2.5], p=0.012), but similar CMAS, and patient/parent scores (Table 3).
Conclusion: The majority of patients achieved medication-free remission. Similar to other JDM cohorts, most were female and young at diagnosis. Sex and race may be important factors as more children who were male or African American did not achieve medication-free remission. Further studies are needed to confirm these findings.
Disclosures: H. Ghumman, None; A. Firdous, None; M. Quinlan-Waters, None; A. Cassedy, None; A. Merritt, None; H. Brunner, Cincinnati Children's Hospital, Pfizer, GlaxoSmithKlein(GSK), AbbVie/Abbott, AstraZeneca, Medimmune, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, EMD Serono, Idorsia, Cerecor, Janssen, Roche, Merck/MSD, Novartis, R-Harm, Sanofi; A. Grom, Sobi, Novartis; D. Lovell, AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKlein(GSK), Novartis, UCB, Bristol-Myers Squibb(BMS), Pfizer, Janssen, NIH/NIAMS, NIH/NICHD, Roche; S. Angeles-Han, None.
Background/Purpose: Juvenile dermatomyositis (JDM) is characterized by symmetric proximal muscle weakness, distinct rash, and a risk for calcinosis and multi-organ involvement. Treatment with systemic immunosuppression is needed. Evidence is limited on the factors associated with disease remission off of medication.
The aims of this study are to describe the demographic, clinical, laboratory, imaging and histological factors in our JDM cohort, and to identify variables associated with medication-free remission.
Methods: This is a retrospective study of a convenience sample of children diagnosed with JDM at ≤18 years of age by a pediatric rheumatologist, with a minimum clinical follow-up of 2 years. Medication-free remission was defined as inactive JDM off all systemic medications for at least 6 months. Medical records were reviewed. We compared the following variables in children who achieved remission to those who did not: demographics, clinical features, muscle enzymes, ANA, myositis-specific auto-antibodies, Childhood Myositis Assessment Scale (CMAS; to assess disease activity), Physician Global Assessment Scores, and Parent/Patient Global Assessment Scores. Group differences were tested using Fisher's Exact tests and Wilcoxon Rank Sum tests. A p< 0.05 was considered statistically significant. Analysis was conducted using SAS 9.3©.
Results: Of 36 participants, 29 (80.5%) achieved medication-free remission while 7 (19.4%) were still on medication at last follow-up. Overall, most were non-Hispanic White (83%), females (69%), had a median age of JDM onset 4 years (range 3-6.5), and diagnosed in the fall (11, 30.6%) and summer (11, 30.5%). Those who achieved remission required treatment for 37 (interquartile [IQR] 27-47) months before discontinuation. Comparing children who achieved medication-free remission to those who did not, median age at JDM onset (4 [IQR 3-6] vs 6 [4-10] years), JDM diagnosis (5 [3-6] v. 8 [4-11] years), and time to treatment initiation (3 [0-14] vs 14 [2-27] days) was not statistically significant. However, more males (71% [n=5] vs. 21% [6], p=0.017) and African Americans (43% [3] vs. 7% [2], p=0.045) did not achieve remission. Further, most of these patients were diagnosed in the fall (43% [3] vs. 28% [8], p=0.034). Both groups had similar presentation of rash, muscle weakness, elevated muscle enzyme levels, ANA positivity, abnormal muscle and skin biopsy, and MRI findings (in those who had them done). 92% were treated with glucocorticoids. Methotrexate (86%) was the most commonly prescribed DMARD. There were no differences in systemic medications given over the disease course between both groups (Table 2). At last follow up, children who did not achieve remission had worse physician global assessment scores (0 [0-0.5] vs 1.5 [0-2.5], p=0.012), but similar CMAS, and patient/parent scores (Table 3).
Conclusion: The majority of patients achieved medication-free remission. Similar to other JDM cohorts, most were female and young at diagnosis. Sex and race may be important factors as more children who were male or African American did not achieve medication-free remission. Further studies are needed to confirm these findings.



Disclosures: H. Ghumman, None; A. Firdous, None; M. Quinlan-Waters, None; A. Cassedy, None; A. Merritt, None; H. Brunner, Cincinnati Children's Hospital, Pfizer, GlaxoSmithKlein(GSK), AbbVie/Abbott, AstraZeneca, Medimmune, Biogen, Boehringer-Ingelheim, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, EMD Serono, Idorsia, Cerecor, Janssen, Roche, Merck/MSD, Novartis, R-Harm, Sanofi; A. Grom, Sobi, Novartis; D. Lovell, AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKlein(GSK), Novartis, UCB, Bristol-Myers Squibb(BMS), Pfizer, Janssen, NIH/NIAMS, NIH/NICHD, Roche; S. Angeles-Han, None.

