Back
Poster Session C
Genetics, genomics and proteomics
Session: (1118–1149) Genetics, Genomics and Proteomics Poster
1125: Genomics of JAK-STAT Signaling in Venous Thromboembolism
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- TK
Tue Kragstrup, MD, PhD
Aarhus University
Aarhus C, Denmark
Abstract Poster Presenter(s)
Stine R Haysen, Ane Langkilde-Lauesen Nielsen, Per Qvist and Tue Kragstrup, Aarhus University, Aarhus, Denmark
Background/Purpose: Janus kinase inhibitors (JAKi) have been associated with an increased risk of venous thromboembolism (VTE). This concern limits the use of JAKi-based therapy. Hence, to improve risk stratification and drug development, it is crucial to understand the possible implication of dysregulated JAK-signal transducers and activators of transcription (STAT) signaling in the pathogenesis of VTE. The objective of this study is to clarify the putative genomic vulnerability to dysregulated JAK-STAT signaling in VTE.
Methods: Here, we systematically mine and analyze large-scale genomic datasets generated from studies comparing VTE patients with healthy controls. First, datasets were divided into 1) single-nucleotide polymorphisms (SNPs) from genome wide associated studies (GWAS), 2) differentially expressed genes (DEGs) from RNAseq and microarrays, and 3) differentially abundant proteins (DAPs) from mass spec proteomics. Then, JAK-STAT pathway genes were defined by Kyoto Encyclopedia of Genes and Genomes (KEGG hsa04630) and available STAT family (STAT1-3) transcription factor binding sites (TFBS) were extracted from the JASPAR database. Finally, Chi-squared test was used to evaluate the representation of genes encoding the JAK-STAT pathway (KEGG) and STAT family TFBS (JASPAR) and their association to VTE (GWAS, DEGs and DAPs). The two bioinformatic tools MAGMA and Ciiider were used to validate the cumulative gene set association, genetic risk burden and molecular characteristics related to JAK-STAT signaling in VTE.
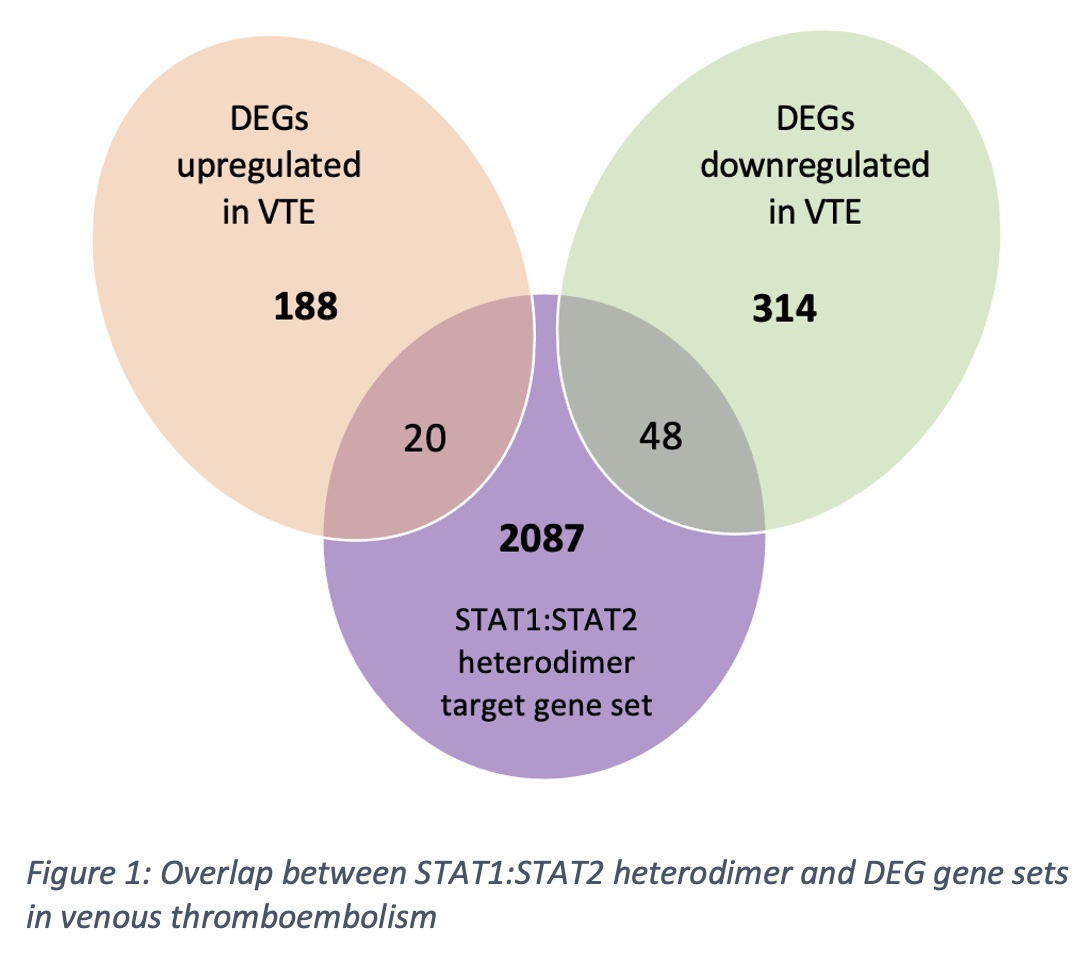
Results: We do not observe a significant overrepresentation of JAK-STAT genes (ntotal=162) among genes annotated to VTE significant GWA loci (ntotal=147, p=0.48). Similarly, the JAK-STAT gene set show no cumulative association to VTE (p=0.98). Applying the same gene set association approach to the STAT target gene sets (ntotal=4570) does not reveal significant association between VTE and STAT1 (noverlap=10, p=0.47), STAT1:STAT2 heterodimer (noverlap=18, p=0.17) and STAT3 (noverlap=6, p=0.20) target gene sets. At the functional molecular level, we do not see any significant overlap between molecules acting in the JAK-STAT pathway and DEGs (ntotal=507, p=0.06) or differentially abundant proteins (DAPs; ntotal=35, p=0.57). However, we observe a significant overlap between downregulated DEGs (ntotal=362) and the STAT1:STAT2 heterodimer target gene set (ntotal= 2155, noverlap=48, p< 0.0001) including downregulation of IL-27RA and CCND3 (Figure 1). Supporting the biological relevance of this finding, we find a weak but statistically significant enrichment of STAT1 TFBS motifs in the promotor sequence of downregulated DEGs compared to non-DEGs (p=0.02).
Conclusion: Here, we provide a coherent approach to assess the genomic basis for the reported association between JAKi treatment and VTE. Our preliminary data suggest that genes under transcriptional control of STAT family TFs may be dysregulated in VTE patients. Obviously, genomic data mining alone cannot guide medical decision making concerning the use of JAKi. However, our results provide a basis for further investigation of adverse events seen with JAKi.
Disclosures: S. Haysen, None; A. Nielsen, None; P. Qvist, None; T. Kragstrup, Aptol Pharma, Pfizer, Bristol-Myers Squibb(BMS), Eli Lilly, Novartis, UCB, AbbVie/Abbott, Gilead.
Background/Purpose: Janus kinase inhibitors (JAKi) have been associated with an increased risk of venous thromboembolism (VTE). This concern limits the use of JAKi-based therapy. Hence, to improve risk stratification and drug development, it is crucial to understand the possible implication of dysregulated JAK-signal transducers and activators of transcription (STAT) signaling in the pathogenesis of VTE. The objective of this study is to clarify the putative genomic vulnerability to dysregulated JAK-STAT signaling in VTE.
Methods: Here, we systematically mine and analyze large-scale genomic datasets generated from studies comparing VTE patients with healthy controls. First, datasets were divided into 1) single-nucleotide polymorphisms (SNPs) from genome wide associated studies (GWAS), 2) differentially expressed genes (DEGs) from RNAseq and microarrays, and 3) differentially abundant proteins (DAPs) from mass spec proteomics. Then, JAK-STAT pathway genes were defined by Kyoto Encyclopedia of Genes and Genomes (KEGG hsa04630) and available STAT family (STAT1-3) transcription factor binding sites (TFBS) were extracted from the JASPAR database. Finally, Chi-squared test was used to evaluate the representation of genes encoding the JAK-STAT pathway (KEGG) and STAT family TFBS (JASPAR) and their association to VTE (GWAS, DEGs and DAPs). The two bioinformatic tools MAGMA and Ciiider were used to validate the cumulative gene set association, genetic risk burden and molecular characteristics related to JAK-STAT signaling in VTE.
Results: We do not observe a significant overrepresentation of JAK-STAT genes (ntotal=162) among genes annotated to VTE significant GWA loci (ntotal=147, p=0.48). Similarly, the JAK-STAT gene set show no cumulative association to VTE (p=0.98). Applying the same gene set association approach to the STAT target gene sets (ntotal=4570) does not reveal significant association between VTE and STAT1 (noverlap=10, p=0.47), STAT1:STAT2 heterodimer (noverlap=18, p=0.17) and STAT3 (noverlap=6, p=0.20) target gene sets. At the functional molecular level, we do not see any significant overlap between molecules acting in the JAK-STAT pathway and DEGs (ntotal=507, p=0.06) or differentially abundant proteins (DAPs; ntotal=35, p=0.57). However, we observe a significant overlap between downregulated DEGs (ntotal=362) and the STAT1:STAT2 heterodimer target gene set (ntotal= 2155, noverlap=48, p< 0.0001) including downregulation of IL-27RA and CCND3 (Figure 1). Supporting the biological relevance of this finding, we find a weak but statistically significant enrichment of STAT1 TFBS motifs in the promotor sequence of downregulated DEGs compared to non-DEGs (p=0.02).
Conclusion: Here, we provide a coherent approach to assess the genomic basis for the reported association between JAKi treatment and VTE. Our preliminary data suggest that genes under transcriptional control of STAT family TFs may be dysregulated in VTE patients. Obviously, genomic data mining alone cannot guide medical decision making concerning the use of JAKi. However, our results provide a basis for further investigation of adverse events seen with JAKi.

Disclosures: S. Haysen, None; A. Nielsen, None; P. Qvist, None; T. Kragstrup, Aptol Pharma, Pfizer, Bristol-Myers Squibb(BMS), Eli Lilly, Novartis, UCB, AbbVie/Abbott, Gilead.

