Back
Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1150–1165) Spondyloarthritis Including PsA – Basic Science Poster
1161: IL-36-receptor Antagonist-Antibodies in Psoriasis and Psoriatic Arthritis
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- MH
Marie-Christin Hoffmann, -None-
Saarland University
Riedlingen, Germany
Abstract Poster Presenter(s)
Marie-Christin Hoffmann1, Natalie Fadle1, Evi Regitz1, Klaus-Dieter Preuss1, Marina Zaks2, Elisabeth Stöger3, Vincent Zimmer4, Philipp Klemm5, Gunter Assmann6, Bernhard Thurner7, Claudia Pföhler8, Joerg Thomas Bittenbring1, Christoph Kessel9 and Lorenz Thurner1, 1José Carreras Center for Immuno- and Gene Therapy and Internal Medicine I, Saarland University Medical School, Homburg/Saar, Germany, 2Department of Nephrology and Internal Intensive Care, Charité University Medicine Berlin, Campus Virchow Clinic, Berlin, Germany, 3Evangelische Kliniken Essen-Mitte gGmbH, Evangelische Huyssens-Stiftung Essen-Huttrop, Essen, Germany, Essen, Germany, 4Department of Medicine, Knappschaftsklinikum Saar, Püttlingen, Germany, Department of Medicine II, Saarland University Medical School, Homburg/Saar, Germany, 5Campus Kerckhoff of Justus Liebig University Giessen, Bad Nauheim, Germany, 6Klinik für Rheumatologie und klinische Immunologie, Mühlenkreiskliniken, Johannes Wesling Klinikum Minden, Minden, Germany, Minden, Germany, 7Department of Pediatrics, Klinikum Kempten, Kempten, Germany, 8Department of Dermatology, Saarland University Medical School, Homburg/Saar, Germany, 9Department of Pediatric Rheumatology and Immunology, University Children’s Hospital Münster, Münster, Germany, Münster, Germany
Background/Purpose: Psoriasis arthritis is known as a subtype of seronegative spondylarthritis. However, autoantibodies against the receptor antagonist at tumor necrosis factor receptor 1 and 2 (TNFR1&2) and death receptor 3 (DR3) Progranulin (PGRN) have been described in psoriatic arthritis.Recently, neutralizing autoantibodies against the interleukin-1 receptor antagonist (IL-1Ra) have been described in severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2)-related diseases, considered to create a pro-inflammatory environment. The interleukin-36 (IL-36) cytokine family is mainly expressed in the skin and, thus, might contribute to the pathogenesis of psoriasis (Ps) and psoriatic arthritis (PsA). In the present study, we screened for hypothetic autoantibodies against the anti-inflammatory mediators IL-36-receptor antagonist (IL-36Ra) and anti-inflammatory IL-38 in PsA, Ps and controls.
Methods: Sera were screened by ELISA for antibodies against IL-36Ra and IL-38. Serum samples of patients with PsA (n=254), Ps (n=102), systemic lupus erythematosus (SLE, n=50), rheumatic arthritis (RA, n=100), ulcerative colitis (UC, n=50), Crohn´s disease (CD, n=50), and healthy controls (HC, n=49) were analyzed. Western blot analysis and isoelectric focusing was performed to detect possible atypical IL-36Ra isoforms. Native western blot was done to detect free IL-36-Ra and possible immunocomplexed IL-36Ra. IL-36Ra serum levels were determined by ELISA.
Results: Anti-IL-36Ra antibodies (Abs) were detected in 5 of 102 (4.9%) patients with Ps, in 12 of 254 (4.7%) patients with PsA and in 1 of 50 (2%) patients with CD. There was no detection of anti-IL-36Ra-antibodies in RA individuals. No anti-IL-38-antibodies could be detected. The antibodies against IL-36Ra belonged to IgG subclass 1, and their titers ranged between 1:200 to 1:1.600. No atypically post-translationally modified isoforms of IL-36Ra have been detected in seropositive patients. The native western blot showed that sera with anti-IL-36Ra-antibodies had less strong bands for free IL-36Ra, but additional bands for IgG-immunocomplexed IL-36Ra. In contrast, seronegative serum samples showed only a band for free IL-36Ra. The concentration of IL-36Ra determined by ELISA was significantly reduced in samples of IL-36Ra-Ab seropositive patients.
Conclusion: Apart from anti-PGRN and anti-IL-1Ra Abs, Abs against IL-36Ra represent a further member of the class of autoantibodies neutralizing anti-inflammatory receptor antagonists. They were found in small subsets of patients with Ps and PsA, where IL-36 imbalances are known for their pathophysiologic impact. Beside PGRN-Abs this is a second proinflammatory autoantibody in PsA.
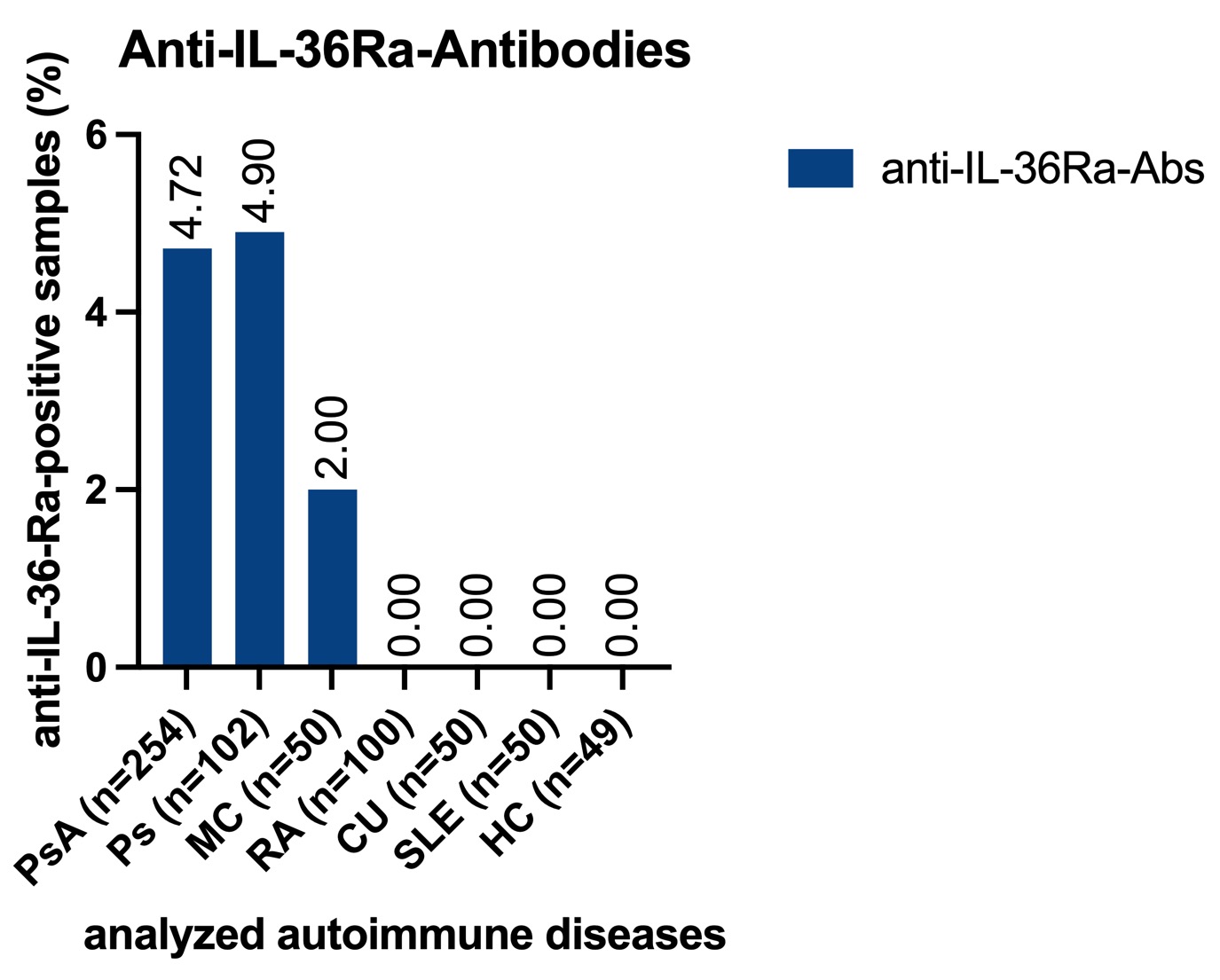 Occurrence of anti-IL-36Ra-antibodies
Occurrence of anti-IL-36Ra-antibodies
.jpg) Anti-IL-36Ra antibody subclasses
Anti-IL-36Ra antibody subclasses
.jpg) Titers of anti-IL-36Ra antibodies
Titers of anti-IL-36Ra antibodies
Disclosures: M. Hoffmann, None; N. Fadle, None; E. Regitz, None; K. Preuss, None; M. Zaks, None; E. Stöger, None; V. Zimmer, None; P. Klemm, None; G. Assmann, None; B. Thurner, Nutricia Milupa, BioNTech; C. Pföhler, None; J. Bittenbring, None; C. Kessel, Novartis, Swedish Orphan Biovitrum (SOBI), Novartis; L. Thurner, Wilhelm Sander-Stiftung, BioNanoMed, Homburger Forschungsförderung programme of the University of Saarland, AbbVie, Janssen, EUSA-Pharm, Takeda, AstraZeneca, Merck, EUSA Pharma.
Background/Purpose: Psoriasis arthritis is known as a subtype of seronegative spondylarthritis. However, autoantibodies against the receptor antagonist at tumor necrosis factor receptor 1 and 2 (TNFR1&2) and death receptor 3 (DR3) Progranulin (PGRN) have been described in psoriatic arthritis.Recently, neutralizing autoantibodies against the interleukin-1 receptor antagonist (IL-1Ra) have been described in severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2)-related diseases, considered to create a pro-inflammatory environment. The interleukin-36 (IL-36) cytokine family is mainly expressed in the skin and, thus, might contribute to the pathogenesis of psoriasis (Ps) and psoriatic arthritis (PsA). In the present study, we screened for hypothetic autoantibodies against the anti-inflammatory mediators IL-36-receptor antagonist (IL-36Ra) and anti-inflammatory IL-38 in PsA, Ps and controls.
Methods: Sera were screened by ELISA for antibodies against IL-36Ra and IL-38. Serum samples of patients with PsA (n=254), Ps (n=102), systemic lupus erythematosus (SLE, n=50), rheumatic arthritis (RA, n=100), ulcerative colitis (UC, n=50), Crohn´s disease (CD, n=50), and healthy controls (HC, n=49) were analyzed. Western blot analysis and isoelectric focusing was performed to detect possible atypical IL-36Ra isoforms. Native western blot was done to detect free IL-36-Ra and possible immunocomplexed IL-36Ra. IL-36Ra serum levels were determined by ELISA.
Results: Anti-IL-36Ra antibodies (Abs) were detected in 5 of 102 (4.9%) patients with Ps, in 12 of 254 (4.7%) patients with PsA and in 1 of 50 (2%) patients with CD. There was no detection of anti-IL-36Ra-antibodies in RA individuals. No anti-IL-38-antibodies could be detected. The antibodies against IL-36Ra belonged to IgG subclass 1, and their titers ranged between 1:200 to 1:1.600. No atypically post-translationally modified isoforms of IL-36Ra have been detected in seropositive patients. The native western blot showed that sera with anti-IL-36Ra-antibodies had less strong bands for free IL-36Ra, but additional bands for IgG-immunocomplexed IL-36Ra. In contrast, seronegative serum samples showed only a band for free IL-36Ra. The concentration of IL-36Ra determined by ELISA was significantly reduced in samples of IL-36Ra-Ab seropositive patients.
Conclusion: Apart from anti-PGRN and anti-IL-1Ra Abs, Abs against IL-36Ra represent a further member of the class of autoantibodies neutralizing anti-inflammatory receptor antagonists. They were found in small subsets of patients with Ps and PsA, where IL-36 imbalances are known for their pathophysiologic impact. Beside PGRN-Abs this is a second proinflammatory autoantibody in PsA.
 Occurrence of anti-IL-36Ra-antibodies
Occurrence of anti-IL-36Ra-antibodies.jpg) Anti-IL-36Ra antibody subclasses
Anti-IL-36Ra antibody subclasses.jpg) Titers of anti-IL-36Ra antibodies
Titers of anti-IL-36Ra antibodiesDisclosures: M. Hoffmann, None; N. Fadle, None; E. Regitz, None; K. Preuss, None; M. Zaks, None; E. Stöger, None; V. Zimmer, None; P. Klemm, None; G. Assmann, None; B. Thurner, Nutricia Milupa, BioNTech; C. Pföhler, None; J. Bittenbring, None; C. Kessel, Novartis, Swedish Orphan Biovitrum (SOBI), Novartis; L. Thurner, Wilhelm Sander-Stiftung, BioNanoMed, Homburger Forschungsförderung programme of the University of Saarland, AbbVie, Janssen, EUSA-Pharm, Takeda, AstraZeneca, Merck, EUSA Pharma.

