Back
Poster Session C
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: (1166–1185) Systemic Sclerosis and Related Disorders – Basic Science Poster
1176: Immunoglobulins G from Systemic Sclerosis Patients May Alter the Secretome of Dermal Fibroblasts
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- AC
Aurélien Chepy, MD
INFINTE U186 inserm, univeristy of Lille
Lille, France
Abstract Poster Presenter(s)
Aurélien Chepy1, Marie Duhamel2, Solange Vivier3, lucile Guilbert3, Eric Hachulla4, Sylvain Dubucquoi3, David Launay3, Michel Salzet2 and Vincent Sobanski3, 1CHU Lille, Département de Médecine Interne et Immunologie Clinique, Centre de Référence des Maladies Auto-immunes Systémiques Rares du Nord et Nord-Ouest de France (CeRAINO), Lille, France, 2Univ. Lille, Inserm, CHU Lille, U1192 - Protéomique Réponse Inflammatoire Spectrométrie de Masse - PRISM, Lille, France, 3Univ. Lille, Inserm, CHU Lille, U1286 - INFINITE - Institute for Translational Research in Inflammation, Lille, France, 4University of Lille, LILLE, France
Background/Purpose: Autoantibodies (Aab) are frequent in systemic sclerosis (SSc).Recently, it has been shown that immunoglobulins G (IgG) from SSc promoted a proinflammatory and profibrotic phenotype in monocytes secretome. Fibroblasts (FB) are key effectors cells in SSc and data on FB proteins secretion in the presence of IgG from SSc patients are lacking. Our objective was to explore the FB secretome in the presence of purified IgG from SSc patients.
Methods: Normal dermal FB were cultured in the presence of purified IgG from patients with diffuse cutaneous SSc (dcSSc) (n=20 [of whom 10 were anti-topoisomerase-I (ATA) positive and 10 were ATA negative]), limited cutaneous SSc anti-centromere positive (lcSSc ACA+) (n=10) or healthy controls (HC (n=10). After 72h of culture, the cell supernatants were collected, centrifuged and passed through a filter to remove the cells. After proteins digestion, secretome was explored using mass spectrometry coupled with liquid chromatography (LC-MS/MS). Analysis of variance (ANOVA) and hierarchical clustering were used to identify proteins responses patterns.
Results: Proteomics identified and quantified 1,268 proteins, among them 377 were significant after ANOVA. SSc and HC secretomes appeared distinct. Hierarchical clustering on significant proteins after ANOVA identified 3 clusters: C1 including mostly dcSSc ATA+ patients, C2 including mostly dcSSc ATA- patients, C3 was more heterogeneous including the majority of HC, lcSSc ACA+ patients and some dcSSc ATA- patients (figure 1). We then studied proteins patterns in the initial groups of subjects. Three main clusters were highlighted. C(a) regrouped 95 proteins which were overexpressed in the presence of purified IgG from dcSSc ATA+ patients (involved in calcium-dependent binding and extracellular matrix constituents). C(b) included 248 proteins mostly underexpressed in the presence of purified IgG from dcSSc ATA+ patients (involved in protein folding and actin filament depolymerization). C(c) gathered 34 proteins overexpressed in presence of purified IgG from all SSc groups (involved in amyloid-beta binding and ECM structural constituents) (figure 2). Finally, we performed differential analyses. Follistatin, amyloid beta A4 protein, myosin-9 and calreticulin were commonly overexpressed in all SSc subtypes. We identified 19 proteins exclusively overexpressed in dcSSc ATA positive patients such as collagen alpha-I type 7 and galectin 3 binding proteins (figure 3).
Conclusion: Using sensitive proteomic approach, we highligthed that purified IgG from SSc patients can modify the FB secretome. Along with similar results on monocyte secretome, these data suggest that SSc-IgG may influence the inflammatory and fibrotic phenotype of different cells.
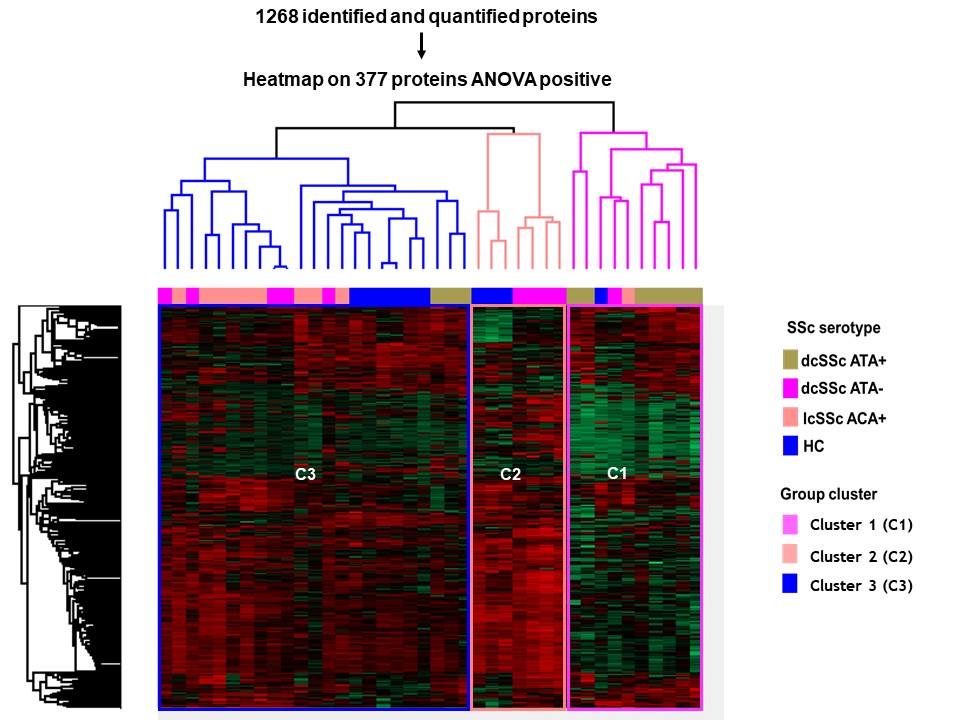 Heatmap representing the 377 differentially expressed proteins after ANOVA in all samples. Cluster analysis identified 3 clusters (C1, C2 and C3).
Heatmap representing the 377 differentially expressed proteins after ANOVA in all samples. Cluster analysis identified 3 clusters (C1, C2 and C3).
IgG HC: purified IgG from healthy controls; IgG dcSSc ATA+: purified IgG from diffuse systemic sclerosis anti-topoisomerase-I positive patients; IgG dcSSc ATA-: purified IgG from diffuse systemic sclerosis anti-topoisomerase-I negative patients; IgG lcSSc ACA+: purified IgG from limited systemic sclerosis anti-centromere positive patients. ANOVA: analysis of variance.
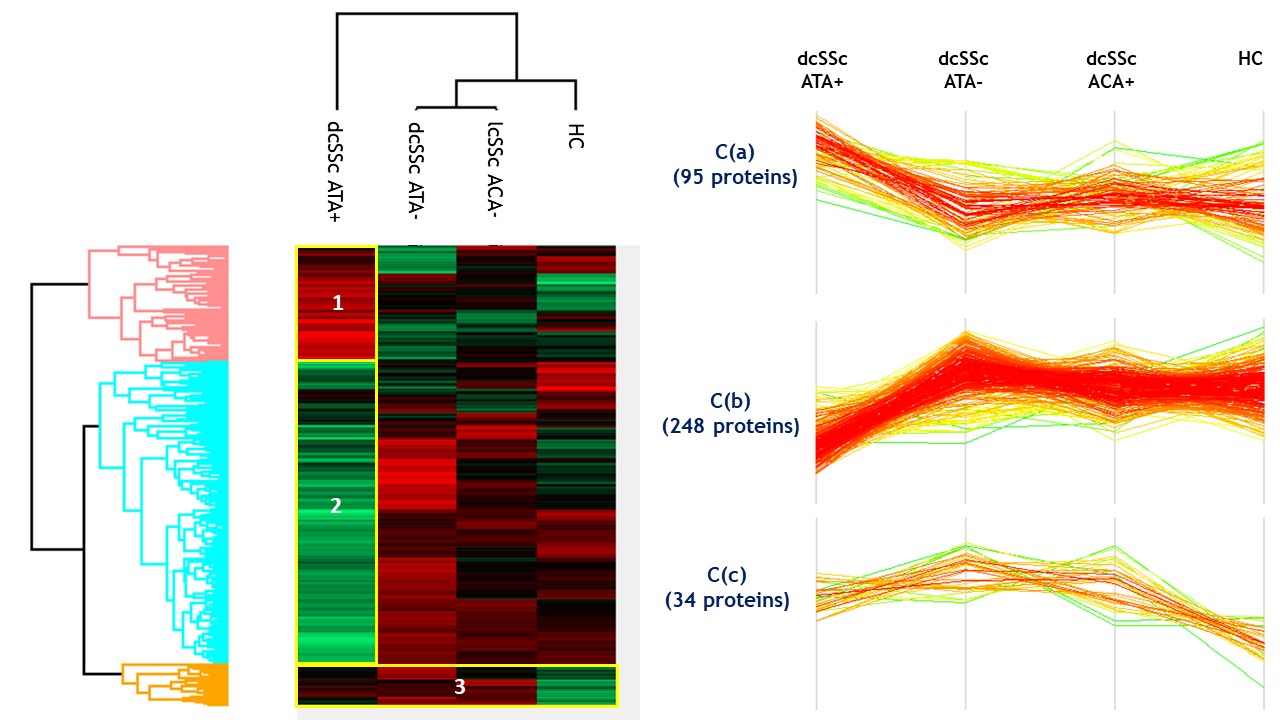 Heatmap representing proteins clusters (C(a), C(b) and C(c)) according to the initial groups of subjects.
Heatmap representing proteins clusters (C(a), C(b) and C(c)) according to the initial groups of subjects.
IgG HC: purified IgG from healthy controls; IgG dcSSc ATA+: purified IgG from diffuse systemic sclerosis anti-topoisomerase-I positive patients; IgG dcSSc ATA-: purified IgG from diffuse systemic sclerosis anti-topoisomerase-I negative patients; IgG lcSSc ACA+: purified IgG from limited systemic sclerosis anti-centromere positive patients.
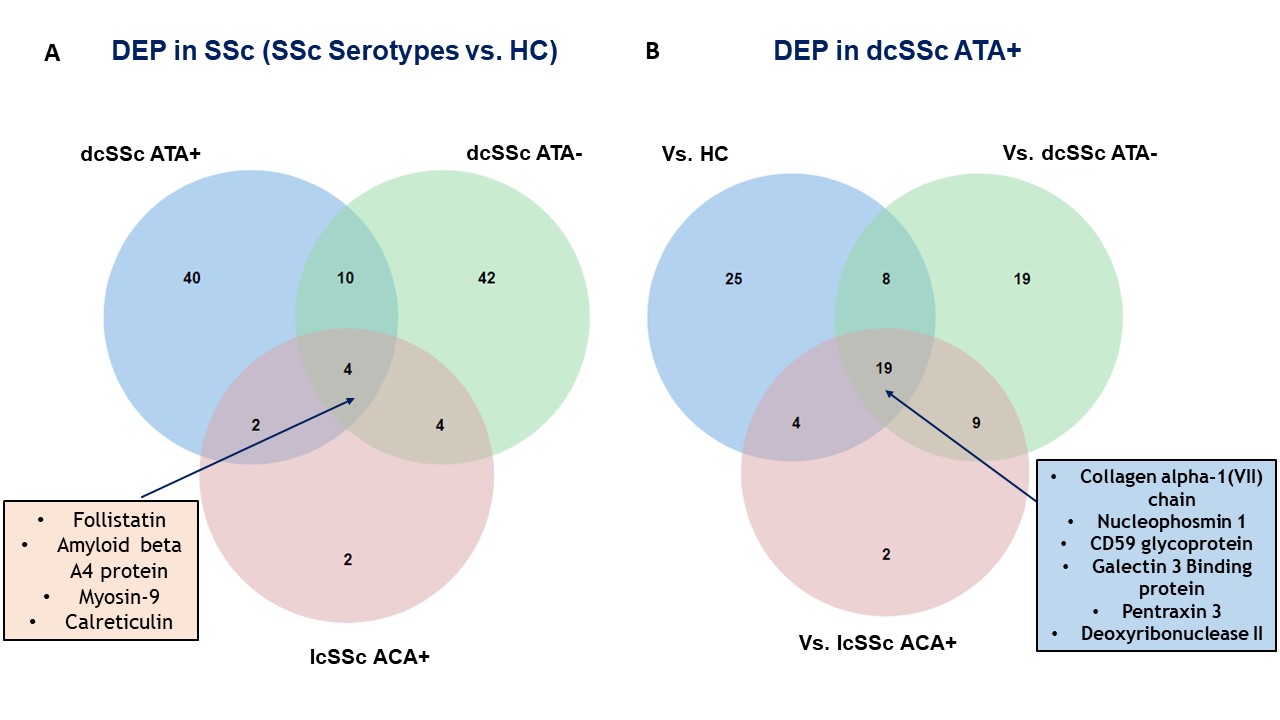 Commonly overexpressed proteins in SSc (all serotypes) (A); Proteins exclusively overexpressed in dcSSc ATA+ (B).
Commonly overexpressed proteins in SSc (all serotypes) (A); Proteins exclusively overexpressed in dcSSc ATA+ (B).
DEP: differentially expressed proteins; SSc: systemic sclerosis; IgG HC: purified IgG from healthy controls; IgG dcSSc ATA+: purified IgG from diffuse systemic sclerosis anti-topoisomerase-I positive patients; IgG dcSSc ATA-: purified IgG from diffuse systemic sclerosis anti-topoisomerase-I negative patients; IgG lcSSc ACA+: purified IgG from limited systemic sclerosis anti-centromere positive patients.
Disclosures: A. Chepy, None; M. Duhamel, None; S. Vivier, None; l. Guilbert, None; E. Hachulla, GlaxoSmithKline, Johnson & Johnson, Roche-Chugai, CSL Behring, Bayer, Boehringer Ingelheim, Sanofi-Genzyme; S. Dubucquoi, None; D. Launay, None; M. Salzet, None; V. Sobanski, None.
Background/Purpose: Autoantibodies (Aab) are frequent in systemic sclerosis (SSc).Recently, it has been shown that immunoglobulins G (IgG) from SSc promoted a proinflammatory and profibrotic phenotype in monocytes secretome. Fibroblasts (FB) are key effectors cells in SSc and data on FB proteins secretion in the presence of IgG from SSc patients are lacking. Our objective was to explore the FB secretome in the presence of purified IgG from SSc patients.
Methods: Normal dermal FB were cultured in the presence of purified IgG from patients with diffuse cutaneous SSc (dcSSc) (n=20 [of whom 10 were anti-topoisomerase-I (ATA) positive and 10 were ATA negative]), limited cutaneous SSc anti-centromere positive (lcSSc ACA+) (n=10) or healthy controls (HC (n=10). After 72h of culture, the cell supernatants were collected, centrifuged and passed through a filter to remove the cells. After proteins digestion, secretome was explored using mass spectrometry coupled with liquid chromatography (LC-MS/MS). Analysis of variance (ANOVA) and hierarchical clustering were used to identify proteins responses patterns.
Results: Proteomics identified and quantified 1,268 proteins, among them 377 were significant after ANOVA. SSc and HC secretomes appeared distinct. Hierarchical clustering on significant proteins after ANOVA identified 3 clusters: C1 including mostly dcSSc ATA+ patients, C2 including mostly dcSSc ATA- patients, C3 was more heterogeneous including the majority of HC, lcSSc ACA+ patients and some dcSSc ATA- patients (figure 1). We then studied proteins patterns in the initial groups of subjects. Three main clusters were highlighted. C(a) regrouped 95 proteins which were overexpressed in the presence of purified IgG from dcSSc ATA+ patients (involved in calcium-dependent binding and extracellular matrix constituents). C(b) included 248 proteins mostly underexpressed in the presence of purified IgG from dcSSc ATA+ patients (involved in protein folding and actin filament depolymerization). C(c) gathered 34 proteins overexpressed in presence of purified IgG from all SSc groups (involved in amyloid-beta binding and ECM structural constituents) (figure 2). Finally, we performed differential analyses. Follistatin, amyloid beta A4 protein, myosin-9 and calreticulin were commonly overexpressed in all SSc subtypes. We identified 19 proteins exclusively overexpressed in dcSSc ATA positive patients such as collagen alpha-I type 7 and galectin 3 binding proteins (figure 3).
Conclusion: Using sensitive proteomic approach, we highligthed that purified IgG from SSc patients can modify the FB secretome. Along with similar results on monocyte secretome, these data suggest that SSc-IgG may influence the inflammatory and fibrotic phenotype of different cells.
 Heatmap representing the 377 differentially expressed proteins after ANOVA in all samples. Cluster analysis identified 3 clusters (C1, C2 and C3).
Heatmap representing the 377 differentially expressed proteins after ANOVA in all samples. Cluster analysis identified 3 clusters (C1, C2 and C3). IgG HC: purified IgG from healthy controls; IgG dcSSc ATA+: purified IgG from diffuse systemic sclerosis anti-topoisomerase-I positive patients; IgG dcSSc ATA-: purified IgG from diffuse systemic sclerosis anti-topoisomerase-I negative patients; IgG lcSSc ACA+: purified IgG from limited systemic sclerosis anti-centromere positive patients. ANOVA: analysis of variance.
 Heatmap representing proteins clusters (C(a), C(b) and C(c)) according to the initial groups of subjects.
Heatmap representing proteins clusters (C(a), C(b) and C(c)) according to the initial groups of subjects. IgG HC: purified IgG from healthy controls; IgG dcSSc ATA+: purified IgG from diffuse systemic sclerosis anti-topoisomerase-I positive patients; IgG dcSSc ATA-: purified IgG from diffuse systemic sclerosis anti-topoisomerase-I negative patients; IgG lcSSc ACA+: purified IgG from limited systemic sclerosis anti-centromere positive patients.
 Commonly overexpressed proteins in SSc (all serotypes) (A); Proteins exclusively overexpressed in dcSSc ATA+ (B).
Commonly overexpressed proteins in SSc (all serotypes) (A); Proteins exclusively overexpressed in dcSSc ATA+ (B). DEP: differentially expressed proteins; SSc: systemic sclerosis; IgG HC: purified IgG from healthy controls; IgG dcSSc ATA+: purified IgG from diffuse systemic sclerosis anti-topoisomerase-I positive patients; IgG dcSSc ATA-: purified IgG from diffuse systemic sclerosis anti-topoisomerase-I negative patients; IgG lcSSc ACA+: purified IgG from limited systemic sclerosis anti-centromere positive patients.
Disclosures: A. Chepy, None; M. Duhamel, None; S. Vivier, None; l. Guilbert, None; E. Hachulla, GlaxoSmithKline, Johnson & Johnson, Roche-Chugai, CSL Behring, Bayer, Boehringer Ingelheim, Sanofi-Genzyme; S. Dubucquoi, None; D. Launay, None; M. Salzet, None; V. Sobanski, None.

