Back
Poster Session C
Vasculitis
Session: (1543â1578) Vasculitis â Non-ANCA-Associated and Related Disorders Poster II
1553: Effectiveness and Safety of Adalimumab versus Leflunomide in Patients with Takayasu Arteritis â a Retrospective Cohort Study
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- Ad
Alexandre de Souza, MD, PhD
UNIFESP-EPM
São Paulo, São Paulo, Brazil
Abstract Poster Presenter(s)
Faustino Peron Filho1, Andressa de Souza Moreira2, Anna Larissa Janes2 and Alexandre Wagner de Souza2, 1UNIFESP-EPM, Mogi das Cruzes, São Paulo, Brazil, 2UNIFESP-EPM, São Paulo, Brazil
Background/Purpose: Therapy for Takayasu arteritis (TAK) is based on the combination of high-dose glucocorticoids and immunosuppressive and/or biologic agents. Patients presenting severe disease manifestations are usually treated with TNF inhibitors (TNFi) even as first-line therapy. However, no comparative studies have been performed to compare the effectiveness of biologic agents versus immunosuppressive drugs in the management of TAK. This study aims to evaluate the one-year effectiveness and safety of adalimumab (ADA) compared to leflunomide (LEF) in TAK patients.
Methods: A retrospective and monocenter cohort study was performed. Inclusion criteria were age ≥ 18 years, fulfillment of the 1990 American College of Rheumatology criteria for TAK, and written informed consent. Among 75 TAK patients under regular follow-up, 44 patients had started either LEF (n = 28) or ADA (n = 16). Patients were evaluated at baseline, at a median of 7.0 months [interquartile range (IQR): 5.0-9.0] (Visit 1) and at 14.0 months (IQR: 13.0-20.0) of follow-up as the final visit. Data regarding disease activity, acute phase reactants, the daily dose of prednisone, side effects, and angiographic progression were collected and analyzed.
Results: LEF and ADA groups had similar features at the baseline visit regarding demographics, disease duration, active disease, levels of acute-phase reactants, therapy before inclusion, and prednisone daily dose, except for the higher frequency of methylprednisolone intravenous pulse therapy in the ADA group (Table 1). In the ADA group, 11 patients (68.8%) used concomitant immunosuppressive agents (i.e., LEF in 7 and methotrexate in 4 patients). At visit 1, median levels of acute-phase reactants and the frequency of complete remission were the same in both groups (i.e., 75%), as well as remission with prednisone < 10mg/day. However, the ADA group had a higher median daily prednisone dose compared to the LEF group (p = 0.014) (Table 2). In the final visit, ADA and LEF groups were comparable for levels of acute-phase reactants, complete remission, daily prednisone dose, and remission with prednisone < 10mg/day. Only patients in the LEF group withdrew from therapy during follow-up (Table 2). Follow-up imaging was available for 35/44 patients. Similar rates of angiographic progression were observed in LEF and ADA groups (40% vs. 35%; p = 0.467). Mild to moderate adverse events were observed only in 5 patients (17.8%) from the LEF group.
Conclusion: LEF and ADA had comparable outcomes after a median of 14.0 months of follow-up. However, withdrawal from therapy and mild-to-moderate adverse events were only observed in the LEF group.
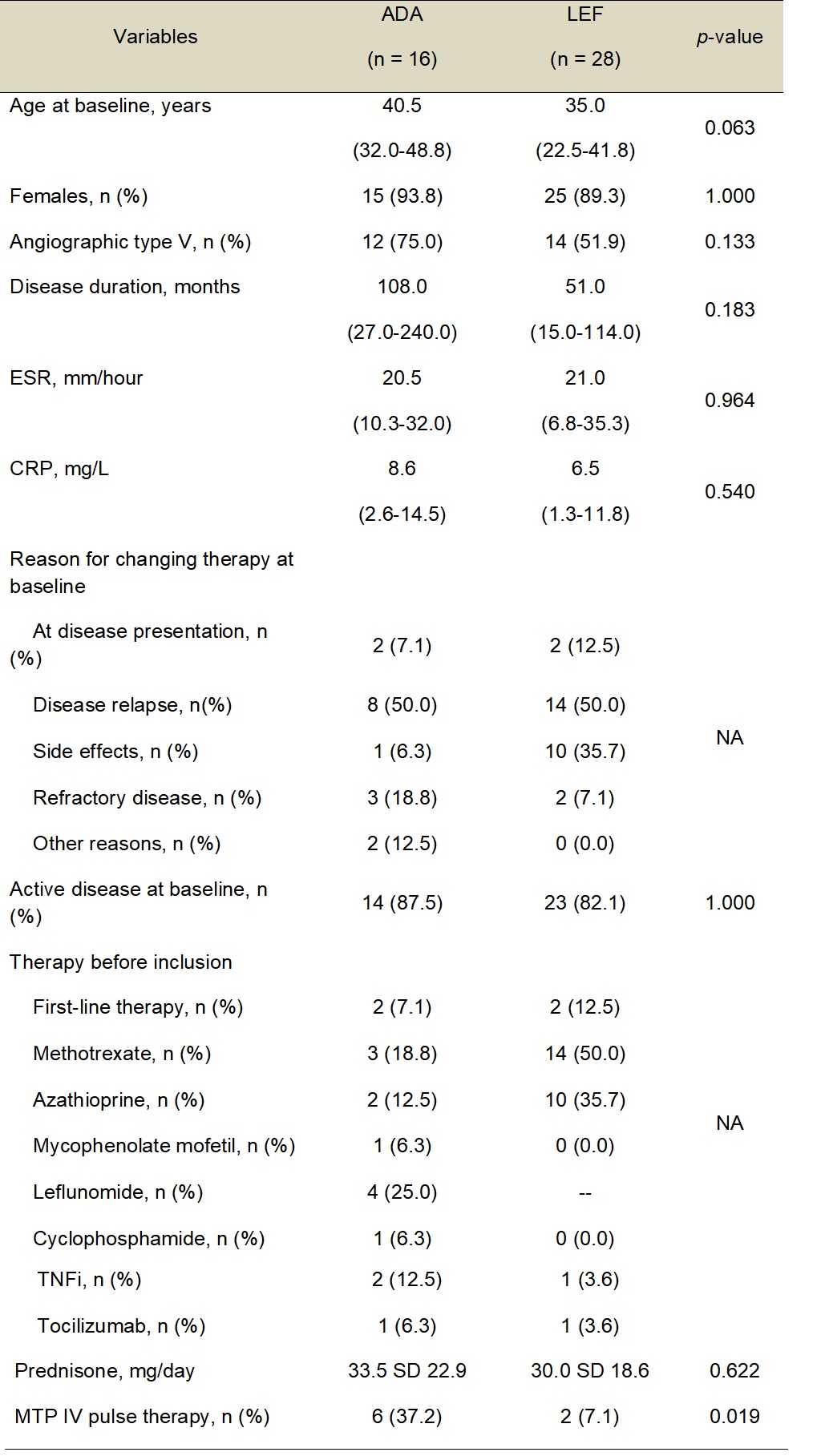 Table 1 â Baseline features of TAK patients under therapy with ADA and LEF.
Table 1 â Baseline features of TAK patients under therapy with ADA and LEF.
ADA â Adalimumab; CRP â C-reactive protein; ESR â Erythrocyte sedimentation rate; IV â Intravenous; LEF â Leflunomide; MTP â Methylprednisolone; n â Number of patients; SD â Standard deviation; TAK â Takayasu arteritis; TNFi â TNF inhibitors.
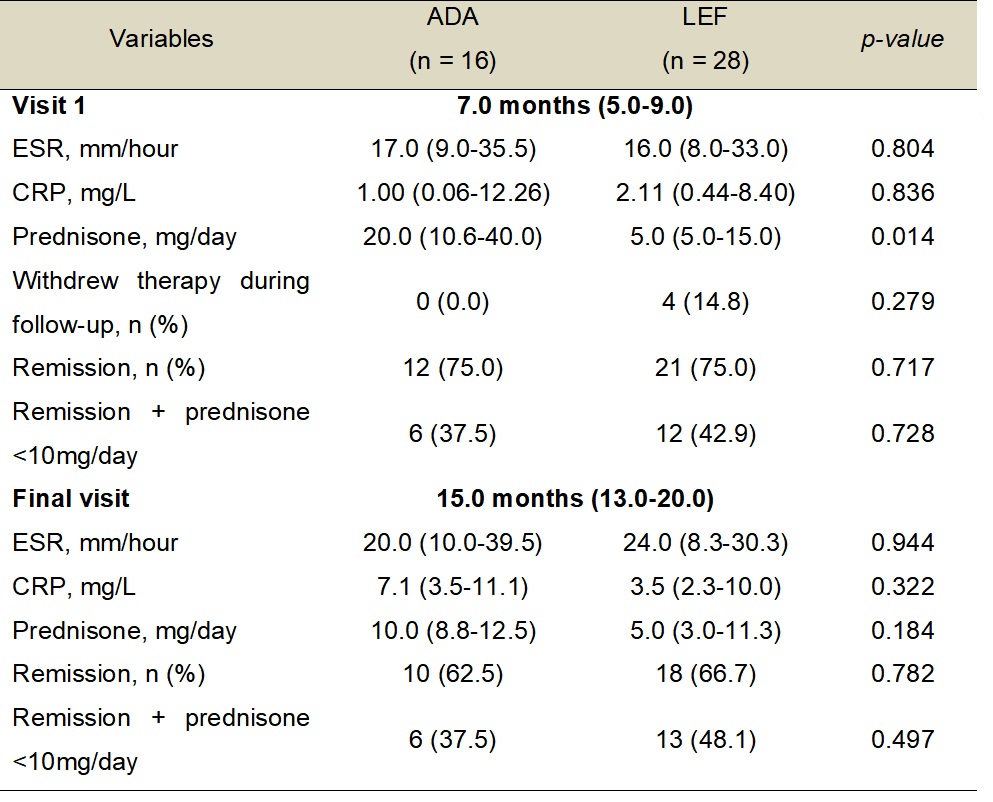 Table 2 â Comparison of outcomes between ADA and LEF groups.
Table 2 â Comparison of outcomes between ADA and LEF groups.
ADA â Adalimumab; CRP â C-reactive protein; ESR â Erythrocyte sedimentation rate; LEF â Leflunomide; n â Number of patients.
Disclosures: F. Peron Filho, None; A. de Souza Moreira, None; A. Janes, None; A. de Souza, None.
Background/Purpose: Therapy for Takayasu arteritis (TAK) is based on the combination of high-dose glucocorticoids and immunosuppressive and/or biologic agents. Patients presenting severe disease manifestations are usually treated with TNF inhibitors (TNFi) even as first-line therapy. However, no comparative studies have been performed to compare the effectiveness of biologic agents versus immunosuppressive drugs in the management of TAK. This study aims to evaluate the one-year effectiveness and safety of adalimumab (ADA) compared to leflunomide (LEF) in TAK patients.
Methods: A retrospective and monocenter cohort study was performed. Inclusion criteria were age ≥ 18 years, fulfillment of the 1990 American College of Rheumatology criteria for TAK, and written informed consent. Among 75 TAK patients under regular follow-up, 44 patients had started either LEF (n = 28) or ADA (n = 16). Patients were evaluated at baseline, at a median of 7.0 months [interquartile range (IQR): 5.0-9.0] (Visit 1) and at 14.0 months (IQR: 13.0-20.0) of follow-up as the final visit. Data regarding disease activity, acute phase reactants, the daily dose of prednisone, side effects, and angiographic progression were collected and analyzed.
Results: LEF and ADA groups had similar features at the baseline visit regarding demographics, disease duration, active disease, levels of acute-phase reactants, therapy before inclusion, and prednisone daily dose, except for the higher frequency of methylprednisolone intravenous pulse therapy in the ADA group (Table 1). In the ADA group, 11 patients (68.8%) used concomitant immunosuppressive agents (i.e., LEF in 7 and methotrexate in 4 patients). At visit 1, median levels of acute-phase reactants and the frequency of complete remission were the same in both groups (i.e., 75%), as well as remission with prednisone < 10mg/day. However, the ADA group had a higher median daily prednisone dose compared to the LEF group (p = 0.014) (Table 2). In the final visit, ADA and LEF groups were comparable for levels of acute-phase reactants, complete remission, daily prednisone dose, and remission with prednisone < 10mg/day. Only patients in the LEF group withdrew from therapy during follow-up (Table 2). Follow-up imaging was available for 35/44 patients. Similar rates of angiographic progression were observed in LEF and ADA groups (40% vs. 35%; p = 0.467). Mild to moderate adverse events were observed only in 5 patients (17.8%) from the LEF group.
Conclusion: LEF and ADA had comparable outcomes after a median of 14.0 months of follow-up. However, withdrawal from therapy and mild-to-moderate adverse events were only observed in the LEF group.
 Table 1 â Baseline features of TAK patients under therapy with ADA and LEF.
Table 1 â Baseline features of TAK patients under therapy with ADA and LEF.ADA â Adalimumab; CRP â C-reactive protein; ESR â Erythrocyte sedimentation rate; IV â Intravenous; LEF â Leflunomide; MTP â Methylprednisolone; n â Number of patients; SD â Standard deviation; TAK â Takayasu arteritis; TNFi â TNF inhibitors.
 Table 2 â Comparison of outcomes between ADA and LEF groups.
Table 2 â Comparison of outcomes between ADA and LEF groups.ADA â Adalimumab; CRP â C-reactive protein; ESR â Erythrocyte sedimentation rate; LEF â Leflunomide; n â Number of patients.
Disclosures: F. Peron Filho, None; A. de Souza Moreira, None; A. Janes, None; A. de Souza, None.

