Back
Poster Session C
Rheumatoid arthritis (RA)
Session: (1417–1439) RA – Treatment Poster III
1422: Incidence and Determinants of Infection in Rheumatoid Arthritis (RA) Patients (Pts) Treated with Subcutaneous Golimumab (GLM) in Canadian Real-World Practice
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- LB
Louis Bessette, MD, MSc
Centre de l'Ostéoporose et de Rhumatologie de Québec
Quebec, QC, Canada
Abstract Poster Presenter(s)
Louis Bessette1, Proton Rahman2, John Kelsall3, Jane Purvis4, Emmanouil Rampakakis5, Allen J. Lehman6, Meagan Rachich7, Francois Nantel8, A. Marilise Marrache9 and Odalis Asin-Milan10, 1Centre de l'Ostoporose et de Rhumatologie de Québec, Québec, QC, Canada, 2Memorial University, St. John's, NL, Canada, 3University of British Columbia, Vancouver, BC, Canada, 4Peterborough Education, Peterborough, ON, Canada, 5McGill University, Department of Pediatrics and JSS Medical Research, Montréal, QC, Canada, 6Janssen Inc., Toronto, ON, Canada, 7Janssen Inc., Guelph, ON, Canada, 8Nantel MedSci Consult, Montréal, QC, Canada, 9Janssen Inc., Dollard-des-Ormeaux, QC, Canada, 10Janssen Canada, Laval, QC, Canada
Background/Purpose: Although biologic use in RA has a well-characterized infections risk factor, most studies were done on 1st-generation anti-tumor necrosis factor (TNFi) drugs or post-drug development (early 2000). This study aimed to describe the long-term incidence of infection of RA pts treated with GLM in routine care, assess the impact of infections on GLM retention, and explore risk factors associated with infection.
Methods: Multicenter, prospective BioTRAC registry collected clinical, laboratory, safety and pt-reported data from TNFi naïve pts or treated with biologics for < 6 months before enrolment. This post-hoc analysis included RA patients who initiated GLM treatment. The incidence density rates (IDR) of total serious (SI) and non-serious (NSI) infections were calculated over 90 months as well as by 6-month interval. Time to first infection and treatment discontinuation was assessed by Kaplan-Meier estimator of survival function. Determinants of infection over time or within the first 6 months were explored using generalized estimating equation models and logistic regression, respectively.
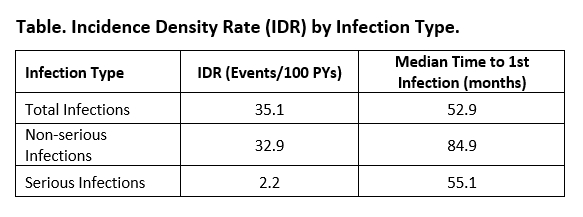
Results: 530 pts were included with a mean (SD) age of 57.7 (13.0) years and disease duration of 8.0 (8.3) years. Of these, 404 (76.2%) were females, 74 (14.0%) were treated with ≤15mg/week MTX, 280 (52.8%) with >15mg/week MTX, while 173 (32.6%) were not on MTX. For corticosteroids (CS), 72 (13.6%) were treated with ≤5mg/day, 63 (11.9%) with >5mg/day, and 391 (73.8%) were not on CS. Diabetes (4.5%), pulmonary disease (8.9%), and renal disease (18.5%) were present. Over a mean follow-up of 27.0 months, the IDR for total infections, NSI, and SI was 35.10 , 32.90, and 2.23 events/100 PYs. Median estimated time to first infection was 52.9 months (SI: 84.9 months; NSI: 55.1 months, Table). Incidence of total infections was 44.0, 37.3, 35.1, 29.4, 31.1, 35.7, 19.3 and 7.4 events/100 PYs at 0-6 months, 6-12 months, 12-24 months, 24-36 months, 36-48 months, 48-60 months, 60-72 months, 72-84 months, respectively and no infections between 84-90 months. For determinants, no significant associations were identified for incidence of infections within the first 6 months, however pulmonary disease was a significant determinant of total infections (OR [95%CI]: 2.19 [1.36-3.52]) and NSI (2.22 [1.35-3.66]) over time, and higher age (1.08 [1.00-1.26]) and high (≥5 mg/day) CS dose (7.25 [1.12-46.80]) were associated with significantly higher odds of SI. For predictors of GLM discontinuation, incidence of SI (6.48 [1.16-36.13]), but not NSI, was associated with significantly higher odds; as well as increased baseline CDAI (1.06 [1.04-1.08]) and use of MTX at low (0.52 [0.30-0.91]) or high dose (0.71 [0.49-1.04]).
Conclusion: Infection rates for this GLM cohort are low compared to rates reported in earlier TNFi studies. Changes over time in pt characteristics starting TNFi (lower disease activity, shorter disease duration, less CS exposure) may explain the decreased risk of infection. Most infections occurred during the first 6 months of treatment and decreased thereafter. Pulmonary disease, higher age and higher CS dose were significant predictors of infections. SIs only were associated with significantly higher odds of treatment discontinuation.
 Incidence Density Rate (IDR) by Infection Type
Incidence Density Rate (IDR) by Infection Type
Disclosures: L. Bessette, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi-Genzyme, UCB, Gilead, Merck/MSD, Organon, Roche; P. Rahman, Janssen, Novartis, AbbVie, Eli Lilly and Company, Pfizer; J. Kelsall, None; J. Purvis, AbbVie, Amgen, BioJAMP, BMS, Gilead, Janssen, Merck, Organon, Pfizer, Roche, Sandoz, Sanofi, Ontario rheumatology association, Ontario Medical Association; E. Rampakakis, Janssen, JSS Medical Research; A. Lehman, Janssen; M. Rachich, Janssen; F. Nantel, Janssen, Johnson & Johnson; A. Marrache, Janssen Pharmaceutical Companies of Johnson & Johnson, Johnson & Johnson; O. Asin-Milan, Janssen.
Background/Purpose: Although biologic use in RA has a well-characterized infections risk factor, most studies were done on 1st-generation anti-tumor necrosis factor (TNFi) drugs or post-drug development (early 2000). This study aimed to describe the long-term incidence of infection of RA pts treated with GLM in routine care, assess the impact of infections on GLM retention, and explore risk factors associated with infection.
Methods: Multicenter, prospective BioTRAC registry collected clinical, laboratory, safety and pt-reported data from TNFi naïve pts or treated with biologics for < 6 months before enrolment. This post-hoc analysis included RA patients who initiated GLM treatment. The incidence density rates (IDR) of total serious (SI) and non-serious (NSI) infections were calculated over 90 months as well as by 6-month interval. Time to first infection and treatment discontinuation was assessed by Kaplan-Meier estimator of survival function. Determinants of infection over time or within the first 6 months were explored using generalized estimating equation models and logistic regression, respectively.
Results: 530 pts were included with a mean (SD) age of 57.7 (13.0) years and disease duration of 8.0 (8.3) years. Of these, 404 (76.2%) were females, 74 (14.0%) were treated with ≤15mg/week MTX, 280 (52.8%) with >15mg/week MTX, while 173 (32.6%) were not on MTX. For corticosteroids (CS), 72 (13.6%) were treated with ≤5mg/day, 63 (11.9%) with >5mg/day, and 391 (73.8%) were not on CS. Diabetes (4.5%), pulmonary disease (8.9%), and renal disease (18.5%) were present. Over a mean follow-up of 27.0 months, the IDR for total infections, NSI, and SI was 35.10 , 32.90, and 2.23 events/100 PYs. Median estimated time to first infection was 52.9 months (SI: 84.9 months; NSI: 55.1 months, Table). Incidence of total infections was 44.0, 37.3, 35.1, 29.4, 31.1, 35.7, 19.3 and 7.4 events/100 PYs at 0-6 months, 6-12 months, 12-24 months, 24-36 months, 36-48 months, 48-60 months, 60-72 months, 72-84 months, respectively and no infections between 84-90 months. For determinants, no significant associations were identified for incidence of infections within the first 6 months, however pulmonary disease was a significant determinant of total infections (OR [95%CI]: 2.19 [1.36-3.52]) and NSI (2.22 [1.35-3.66]) over time, and higher age (1.08 [1.00-1.26]) and high (≥5 mg/day) CS dose (7.25 [1.12-46.80]) were associated with significantly higher odds of SI. For predictors of GLM discontinuation, incidence of SI (6.48 [1.16-36.13]), but not NSI, was associated with significantly higher odds; as well as increased baseline CDAI (1.06 [1.04-1.08]) and use of MTX at low (0.52 [0.30-0.91]) or high dose (0.71 [0.49-1.04]).
Conclusion: Infection rates for this GLM cohort are low compared to rates reported in earlier TNFi studies. Changes over time in pt characteristics starting TNFi (lower disease activity, shorter disease duration, less CS exposure) may explain the decreased risk of infection. Most infections occurred during the first 6 months of treatment and decreased thereafter. Pulmonary disease, higher age and higher CS dose were significant predictors of infections. SIs only were associated with significantly higher odds of treatment discontinuation.
 Incidence Density Rate (IDR) by Infection Type
Incidence Density Rate (IDR) by Infection TypeDisclosures: L. Bessette, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi-Genzyme, UCB, Gilead, Merck/MSD, Organon, Roche; P. Rahman, Janssen, Novartis, AbbVie, Eli Lilly and Company, Pfizer; J. Kelsall, None; J. Purvis, AbbVie, Amgen, BioJAMP, BMS, Gilead, Janssen, Merck, Organon, Pfizer, Roche, Sandoz, Sanofi, Ontario rheumatology association, Ontario Medical Association; E. Rampakakis, Janssen, JSS Medical Research; A. Lehman, Janssen; M. Rachich, Janssen; F. Nantel, Janssen, Johnson & Johnson; A. Marrache, Janssen Pharmaceutical Companies of Johnson & Johnson, Johnson & Johnson; O. Asin-Milan, Janssen.

