Back
Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1486–1517) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster III
1488: Incremental Healthcare Resource Utilization and Costs of Herpes Zoster in Patients with Psoriatic Arthritis: A Retrospective Cohort Study
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
.jpg)
David Singer, PharmD, MSc
Director, Health Outcomes and Epidemiology
GSK
Brooklyn, NY, United States
Abstract Poster Presenter(s)
David Singer1, Philippe Thompson-Leduc2, Siyu Ma3, Deepshekhar Gupta4, Wendy Cheng5, Selvam Sendhil6, Manasvi Sundar6, Ella Hagopian5, Mei Sheng Duh7 and Sara Poston1, 1GSK, Philadelphia, PA, 2Analysis Group, Inc., Montréal, QC, Canada, 3GSK/Tufts Medical Center, Boston, MA, 4Analysis Group, Inc., Menlo Park, CA, 5Analysis Group, Inc., Boston, MA, 6Analysis Group, Inc., Los Angeles, CA, 7Analysis Group Inc, Boston, MA
Background/Purpose: Patients with psoriatic arthritis (PsA) may be at increased risk of herpes zoster (HZ). HZ can involve a painful dermatomal rash and lead to significant complications. This study estimated incremental healthcare resource utilization (HRU) and costs associated with HZ in patients with PsA.
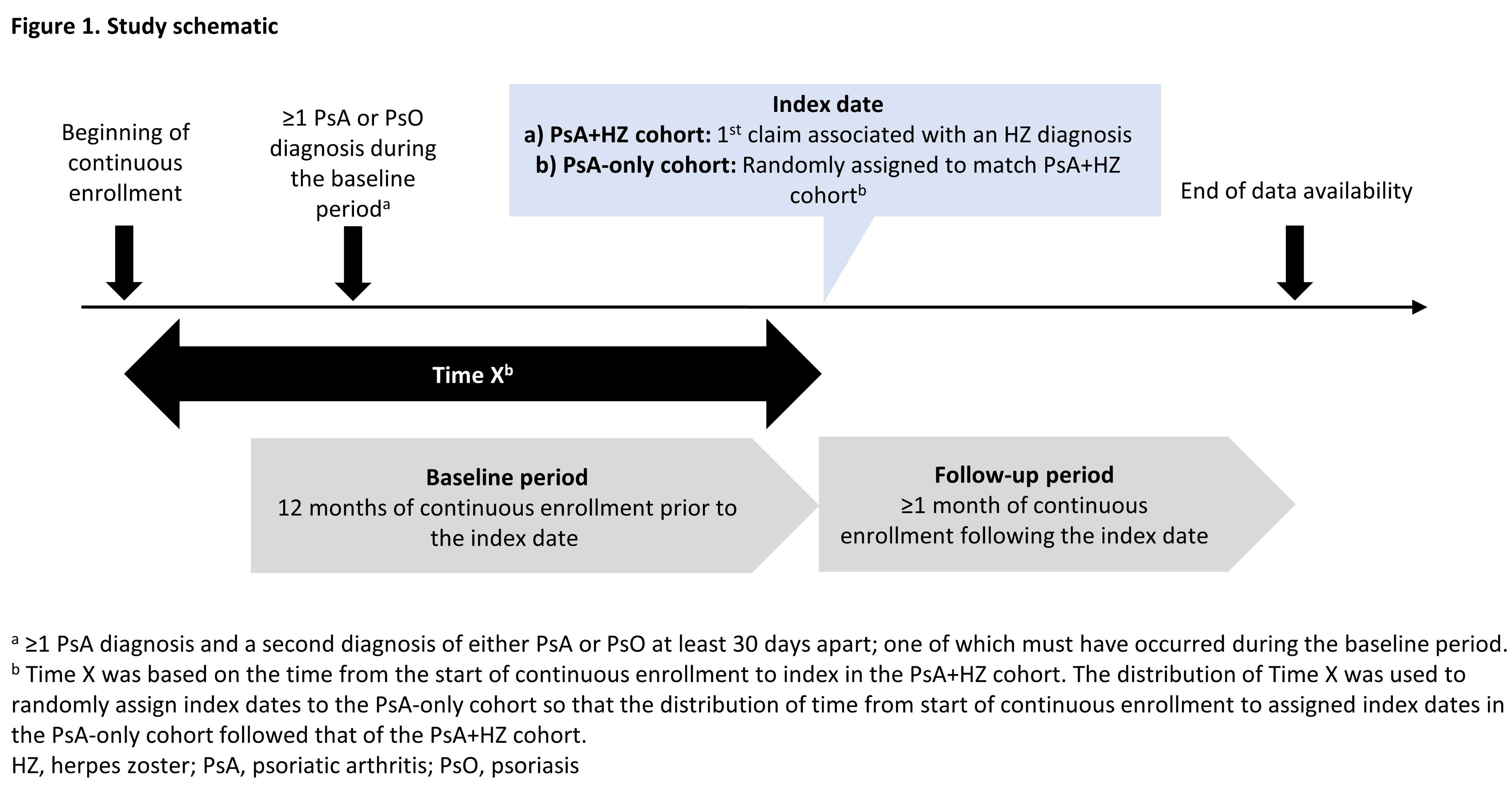
Methods: This retrospective cohort study used administrative claims data from Oct 1, 2015 to Feb 28, 2020 that included patients with Medicare Advantage with Part D or commercial insurance. Adults (age ≥18 years) with PsA were identified using International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes, requiring ≥1 diagnosis (dx) of PsA (L40.5) and a second dx of either PsA or PsO (L40.0-L40.4, L40.8-L40.9) at least 30 days apart, and were assigned to cohorts based on the presence of an HZ dx after the first PsA dx. Index date was the first observed HZ dx (for the PsA+HZ cohort) or was assigned (for the PsA-only cohort) based on the distribution of time from the start of continuous enrollment (CE) to index dates in the PsA+HZ cohort. All patients were required to have 12 months of CE pre-index (baseline) and ≥1 month of CE post-index (follow-up); ≥1 PsA or PsO dx must have occurred during baseline (Figure 1). Patients with a HZ vaccination during baseline or on index or HZ dx prior to index were excluded. Clinical and demographic characteristics were measured during baseline; outcomes, including number of HRU encounters and costs were measured during follow-up at 1 month post-index and, for those with sufficient follow-up time, at 3 months post-index. Propensity scores were calculated using logistic regression with cohort as the outcome and baseline characteristics as predictors. Outcomes were compared between cohorts using generalized linear models, adjusting for differences in baseline characteristics and propensity score, in a doubly robust approach.
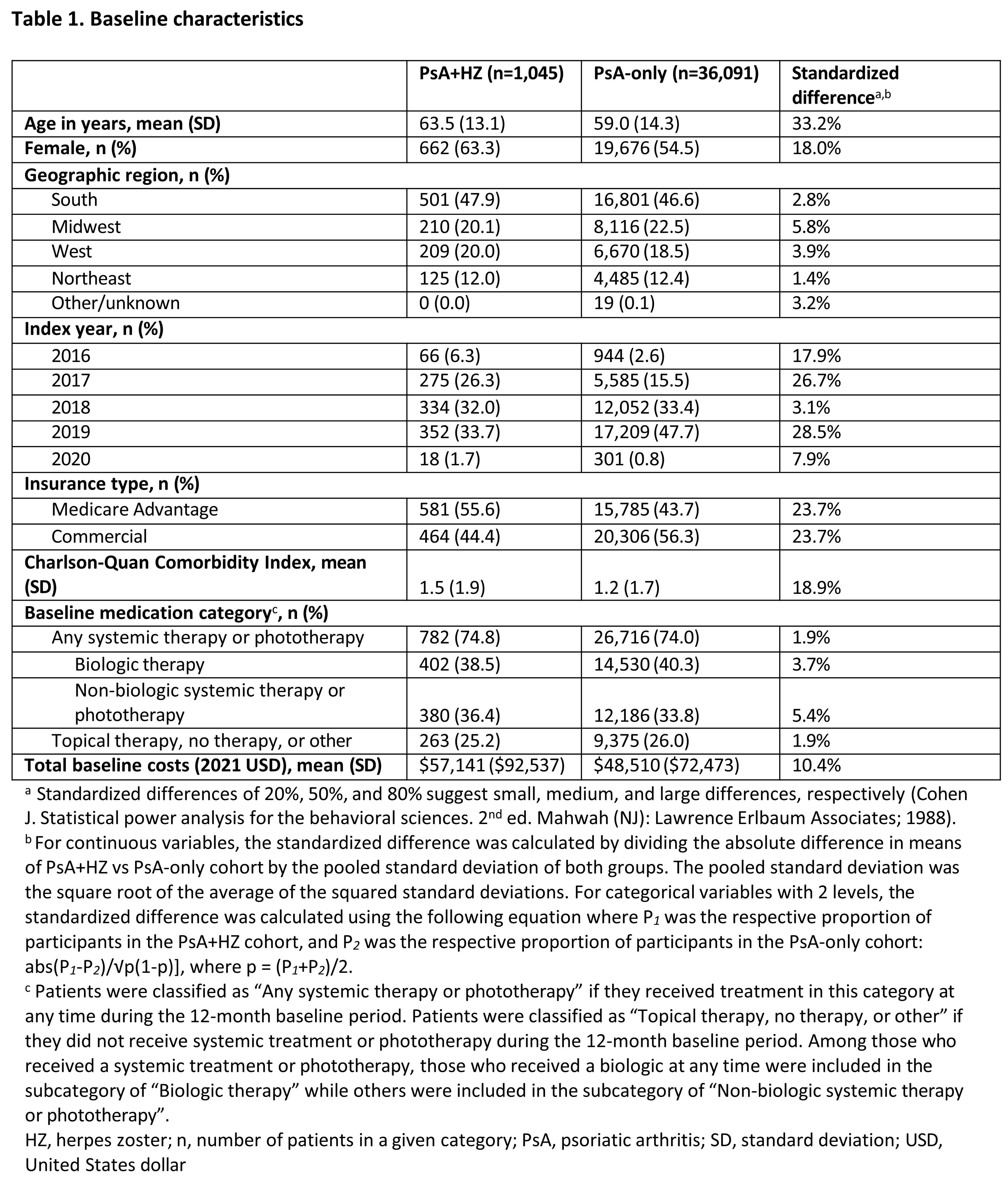
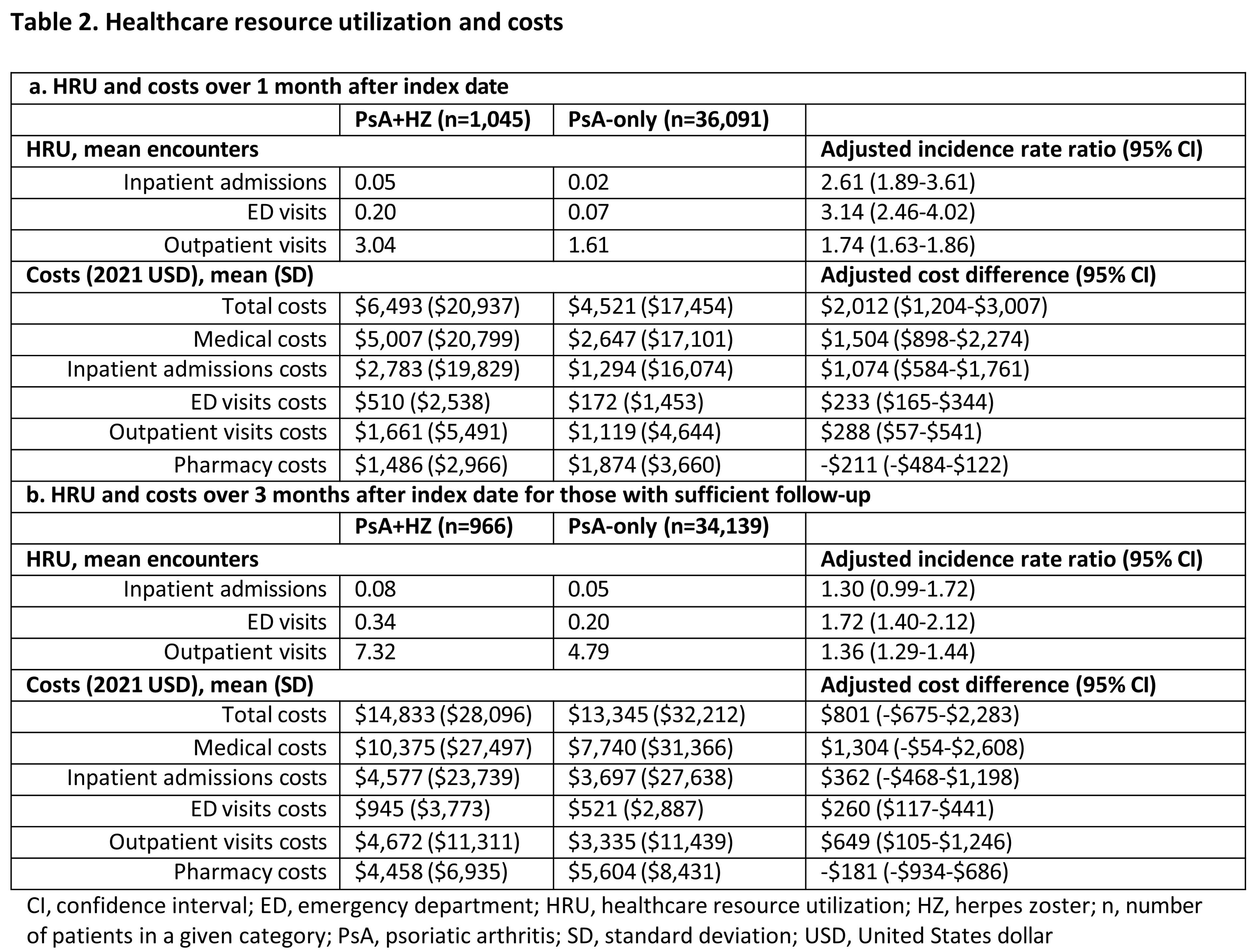
Results: The PsA+HZ and PsA-only cohorts included 1,045 and 36,091 patients, respectively. PsA+HZ and PsA-only cohorts had mean (standard deviation) ages of 63.5 (13.1) and 59.0 (14.3) years and 74-75% of patients received systemic therapy or phototherapy during baseline across both cohorts (Table 1). In the month after index, there was a higher rate of HRU in the PsA+HZ vs PsA-only cohort; adjusted incidence rate ratios for inpatient, emergency department, and outpatient visits were 2.61 (95% confidence interval [CI] 1.89-3.61), 3.14 (95% CI: 2.46-4.02), and 1.74 (95% CI: 1.63-1.86), respectively, for PsA+HZ vs PsA-only cohort. The mean adjusted total, medical, and inpatient cost differences between the PsA+HZ and PsA-only cohorts 1 month after index were $2,012 (95% CI: $1,204-$3,007), $1,504 (95% CI: $898-$2,274), and $1,074 (95% CI: $584-$1,761), respectively. HRU and costs were numerically higher for the PsA+HZ vs PsA-only cohort at 3 months (Table 2).
Conclusion: Patients with PsA and HZ had higher HRU and costs than those without HZ, suggesting a need to consider measures to prevent HZ in this population.
Acknowledgments: Authors would like to thank Business & Decision Life Sciences platform for editorial assistance and publication coordination, on behalf of GSK. Grégory Leroux coordinated publication development and editorial support.
Disclosures: D. Singer, GSK group of companies, Boehringer Ingelheim Pharmaceuticals, Inc; P. Thompson-Leduc, GSK group of companies; S. Ma, GSK group of companies; D. Gupta, GSK group of companies; W. Cheng, GSK group of companies; S. Sendhil, GSK group of companies; M. Sundar, GSK group of companies; E. Hagopian, GSK group of companies; M. Duh, Analysis Group, GlaxoSmithKline (GSK); S. Poston, GSK group of companies.
Background/Purpose: Patients with psoriatic arthritis (PsA) may be at increased risk of herpes zoster (HZ). HZ can involve a painful dermatomal rash and lead to significant complications. This study estimated incremental healthcare resource utilization (HRU) and costs associated with HZ in patients with PsA.
Methods: This retrospective cohort study used administrative claims data from Oct 1, 2015 to Feb 28, 2020 that included patients with Medicare Advantage with Part D or commercial insurance. Adults (age ≥18 years) with PsA were identified using International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes, requiring ≥1 diagnosis (dx) of PsA (L40.5) and a second dx of either PsA or PsO (L40.0-L40.4, L40.8-L40.9) at least 30 days apart, and were assigned to cohorts based on the presence of an HZ dx after the first PsA dx. Index date was the first observed HZ dx (for the PsA+HZ cohort) or was assigned (for the PsA-only cohort) based on the distribution of time from the start of continuous enrollment (CE) to index dates in the PsA+HZ cohort. All patients were required to have 12 months of CE pre-index (baseline) and ≥1 month of CE post-index (follow-up); ≥1 PsA or PsO dx must have occurred during baseline (Figure 1). Patients with a HZ vaccination during baseline or on index or HZ dx prior to index were excluded. Clinical and demographic characteristics were measured during baseline; outcomes, including number of HRU encounters and costs were measured during follow-up at 1 month post-index and, for those with sufficient follow-up time, at 3 months post-index. Propensity scores were calculated using logistic regression with cohort as the outcome and baseline characteristics as predictors. Outcomes were compared between cohorts using generalized linear models, adjusting for differences in baseline characteristics and propensity score, in a doubly robust approach.
Results: The PsA+HZ and PsA-only cohorts included 1,045 and 36,091 patients, respectively. PsA+HZ and PsA-only cohorts had mean (standard deviation) ages of 63.5 (13.1) and 59.0 (14.3) years and 74-75% of patients received systemic therapy or phototherapy during baseline across both cohorts (Table 1). In the month after index, there was a higher rate of HRU in the PsA+HZ vs PsA-only cohort; adjusted incidence rate ratios for inpatient, emergency department, and outpatient visits were 2.61 (95% confidence interval [CI] 1.89-3.61), 3.14 (95% CI: 2.46-4.02), and 1.74 (95% CI: 1.63-1.86), respectively, for PsA+HZ vs PsA-only cohort. The mean adjusted total, medical, and inpatient cost differences between the PsA+HZ and PsA-only cohorts 1 month after index were $2,012 (95% CI: $1,204-$3,007), $1,504 (95% CI: $898-$2,274), and $1,074 (95% CI: $584-$1,761), respectively. HRU and costs were numerically higher for the PsA+HZ vs PsA-only cohort at 3 months (Table 2).
Conclusion: Patients with PsA and HZ had higher HRU and costs than those without HZ, suggesting a need to consider measures to prevent HZ in this population.
Acknowledgments: Authors would like to thank Business & Decision Life Sciences platform for editorial assistance and publication coordination, on behalf of GSK. Grégory Leroux coordinated publication development and editorial support.



Disclosures: D. Singer, GSK group of companies, Boehringer Ingelheim Pharmaceuticals, Inc; P. Thompson-Leduc, GSK group of companies; S. Ma, GSK group of companies; D. Gupta, GSK group of companies; W. Cheng, GSK group of companies; S. Sendhil, GSK group of companies; M. Sundar, GSK group of companies; E. Hagopian, GSK group of companies; M. Duh, Analysis Group, GlaxoSmithKline (GSK); S. Poston, GSK group of companies.

