Back
Poster Session C
Genetics, genomics and proteomics
Session: (1118–1149) Genetics, Genomics and Proteomics Poster
1131: Integrating Genome and Transcriptome-Wide Associations with Real-World Data to Assess Risk for Leukopenia in Patients Taking Azathioprine
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Cecilia Chung, MD, MPH
Vanderbilt University Medical Center
Nashville, TN, United States
Abstract Poster Presenter(s)
Puran Nepal, Jacy Zanussi, Alyson Dickson, Laura Daniel, Tyne Miller-Fleming, Peter Straub, Adriana Hung, Wei-Qi Wei, Nancy Cox, Michael Stein, QiPing Feng and Cecilia Chung, Vanderbilt University Medical Center, Nashville, TN
Background/Purpose: Azathioprine is used to treat several autoimmune conditions, but its use is limited by side effects. Leukopenia, a severe, potentially life-threatening side effect is associated with genetic variants in TPMT and NUDT15 ascribed to poor or intermediate metabolizer phenotypes. However, most patients who develop leukopenia while taking azathioprine do not carry these genetic variants. We hypothesized that models developed combining clinical, genomic, and virtual transcriptomic data could assess the risk for leukopenia among normal TPMT and NUDT15 metabolizers who take azathioprine for autoimmune and other inflammatory conditions.
Methods: Using de-identified electronic health records (EHRs) linked to a DNA biobank, we built a cohort of new users of azathioprine with EHR-reported White race and normal TPMT and NUDT15 metabolizer status. We excluded patients whose primary indication for azathioprine was organ transplant. Leukopenia was defined as a WBC count <3000/µL during azathioprine exposure. We randomly segmented the dataset into a training set (70%) and a testing set (30%) and ran a Ridge regression for leukopenia in the training set that included the following variables: sex, age, initial dose, primary indication for azathioprine, baseline WBC count, whether patients were tested for TPMT before initiation, concurrent use of xanthine oxidase inhibitors and other immunosuppressants, the top 10 SNPs identified from a prior GWAS (rs45566135, rs11664064, rs77179135, rs61913144, rs185367737, rs111703990, rs181895696, rs76996495, rs74550188, rs662061), and the genetically predicted expression of a gene associated with azathioprine-induced leukopenia (MFHAS1) in a prior transcriptome wide-association study. Second, we used the coefficients from this model to build a score to discriminate patients who developed leukopenia while taking azathioprine and applied it to both the training and testing sets. Finally, we created score tertiles based on the training set and assessed risk between tertiles in both the training and testing sets.
Results: Among 1184 azathioprine users (64.3% female, mean age 43.7±16.7) followed over a median of 18.8 months, 125 patients developed leukopenia. Scores developed with the coefficients from the Ridge regressions performed well with receiver operating characteristic curves (ROCs). The area under the ROC curves (AUCs) for the training and testing sets were 0.84 (95% C.I. 0.80-0.89) and 0.79 (95% C.I. 0.70-0.87), respectively. When we classified patients into high, intermediate, and low scores, based on tertiles with cut-offs defined from the training set, the risk for leukopenia was higher among patients with scores in the highest tertile compared to the lowest tertile for both the training and testing sets: HR=13.44 (95% C.I. 4.15-43.50, p< 0.001) and HR=7.58 (95% C.I. 2.25-25.52, p< 0.001), respectively (Figure).
Conclusion: A score including real-world, genomic and virtual transcriptome data discriminates normal TPMT and NUDT15 metabolizers at highest risk to develop leukopenia while taking azathioprine for inflammatory conditions. Future studies with external validation of our findings are warranted.
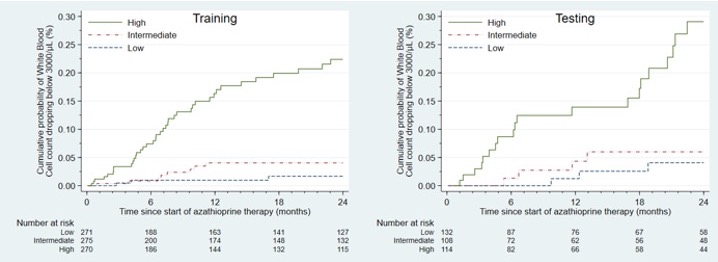 Association between a score combining genetic and clinical data and cumulative incidence of leukopenia
Association between a score combining genetic and clinical data and cumulative incidence of leukopenia
Disclosures: P. Nepal, None; J. Zanussi, None; A. Dickson, None; L. Daniel, None; T. Miller-Fleming, None; P. Straub, None; A. Hung, None; W. Wei, None; N. Cox, None; M. Stein, None; Q. Feng, None; C. Chung, None.
Background/Purpose: Azathioprine is used to treat several autoimmune conditions, but its use is limited by side effects. Leukopenia, a severe, potentially life-threatening side effect is associated with genetic variants in TPMT and NUDT15 ascribed to poor or intermediate metabolizer phenotypes. However, most patients who develop leukopenia while taking azathioprine do not carry these genetic variants. We hypothesized that models developed combining clinical, genomic, and virtual transcriptomic data could assess the risk for leukopenia among normal TPMT and NUDT15 metabolizers who take azathioprine for autoimmune and other inflammatory conditions.
Methods: Using de-identified electronic health records (EHRs) linked to a DNA biobank, we built a cohort of new users of azathioprine with EHR-reported White race and normal TPMT and NUDT15 metabolizer status. We excluded patients whose primary indication for azathioprine was organ transplant. Leukopenia was defined as a WBC count <3000/µL during azathioprine exposure. We randomly segmented the dataset into a training set (70%) and a testing set (30%) and ran a Ridge regression for leukopenia in the training set that included the following variables: sex, age, initial dose, primary indication for azathioprine, baseline WBC count, whether patients were tested for TPMT before initiation, concurrent use of xanthine oxidase inhibitors and other immunosuppressants, the top 10 SNPs identified from a prior GWAS (rs45566135, rs11664064, rs77179135, rs61913144, rs185367737, rs111703990, rs181895696, rs76996495, rs74550188, rs662061), and the genetically predicted expression of a gene associated with azathioprine-induced leukopenia (MFHAS1) in a prior transcriptome wide-association study. Second, we used the coefficients from this model to build a score to discriminate patients who developed leukopenia while taking azathioprine and applied it to both the training and testing sets. Finally, we created score tertiles based on the training set and assessed risk between tertiles in both the training and testing sets.
Results: Among 1184 azathioprine users (64.3% female, mean age 43.7±16.7) followed over a median of 18.8 months, 125 patients developed leukopenia. Scores developed with the coefficients from the Ridge regressions performed well with receiver operating characteristic curves (ROCs). The area under the ROC curves (AUCs) for the training and testing sets were 0.84 (95% C.I. 0.80-0.89) and 0.79 (95% C.I. 0.70-0.87), respectively. When we classified patients into high, intermediate, and low scores, based on tertiles with cut-offs defined from the training set, the risk for leukopenia was higher among patients with scores in the highest tertile compared to the lowest tertile for both the training and testing sets: HR=13.44 (95% C.I. 4.15-43.50, p< 0.001) and HR=7.58 (95% C.I. 2.25-25.52, p< 0.001), respectively (Figure).
Conclusion: A score including real-world, genomic and virtual transcriptome data discriminates normal TPMT and NUDT15 metabolizers at highest risk to develop leukopenia while taking azathioprine for inflammatory conditions. Future studies with external validation of our findings are warranted.
 Association between a score combining genetic and clinical data and cumulative incidence of leukopenia
Association between a score combining genetic and clinical data and cumulative incidence of leukopeniaDisclosures: P. Nepal, None; J. Zanussi, None; A. Dickson, None; L. Daniel, None; T. Miller-Fleming, None; P. Straub, None; A. Hung, None; W. Wei, None; N. Cox, None; M. Stein, None; Q. Feng, None; C. Chung, None.

