Back
Poster Session C
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: (1518–1542) Systemic Sclerosis and Related Disorders – Clinical Poster II
1521: Long-term Follow-Up of Participants of the Phase II Study That Evaluated Subcutaneous Abatacept vs. Placebo in Diffuse Cutaneous Systemic Sclerosis (ASSET Trial)
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Dinesh Khanna, MD, MSc
University of Michigan
Ann Arbor, MI, United States
Abstract Poster Presenter(s)
Nadine Sarsam1, Sindhu R. Johnson2, Lorinda Chung3, Suzanne Kafaja4, Janet Pope5, Robyn Domsic6, Maureen Mayes7, Nora Sandorfi8, Virginia Steen9, Flavia Castelino10, Faye Hant11, Suiyuan Huang1 and Dinesh Khanna12, 1University of Michigan, Ann Arbor, MI, 2Division of Rheumatology, Department of Medicine, Schroeder Arthritis Institute, Krembil Research Institute, Toronto Western and Mount Sinai Hospitals; Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, ON, Canada, 3Stanford University, Stanford, CA, 4UCLA Department of Medicine, Division of Rheumatology, Los Angeles, CA, 5University of Western Ontario, London, ON, Canada, 6University of Pittsburgh, Pittsburgh, PA, 7Division of Rheumatology and Clinical Immunogenetics, University of Texas McGovern Medical School, Houston, TX, 8University of Pennsylvania, Philadelphia, PA, 9Georgetown University School of Medicine, Washington, DC, 10Massachusetts General Hospital, Boston, MA, 11Medical University of South Carolina, Charleston, SC, 12Division of Rheumatology, Department of Internal Medicine, Scleroderma Program, University of Michigan, Ann Arbor, MI
Background/Purpose: Abatacept (ABA) is a cytotoxic T-lymphocyte-associated molecule-4 immunoglobulin fusion protein which has been shown to induce regression of dermal fibrosis in patients with systemic sclerosis (SSc). We previously conducted a 12-month double-blind phase II trial with either ABA or placebo1 followed by a 6-month open-label extension (OLE) with ABA2. This ongoing long term follow up study (LTFU) assessed longer term outcomes, including safety and efficacy of the use of weekly 125 mg subcutaneous ABA in dcSSc, with insights into trends in skin score, functional limitations, and internal organ involvement.
Methods: The current study is ongoing at 11 US and Canadian sites. Inclusion criteria included any participants from the ASSET trial who had participated in the OLE. Each participant signed a new IRB approved consent form. Participants were not required to be on ABA and could be on other immunosuppressive therapies, including investigational agents. The study included two collection time points per year. The outcomes included safety of ABA and change in mRSS (ΔmRSS), ΔHAQ-DI, Δ patient and physician global assessments, and ΔFVC% from the last available assessment in OLE. Mean and standard deviation were reported for efficacy data, and T-test was performed for the difference between ABA and non ABA groups during the LTFU.
Results: 48 patients consented in the ASSET LTFU (16 continued on ABA and 32 on other medications). 44 (92%) participants are continuing in the LTFU. Median time on ABA since the start of LTFU is 1434 days. At baseline of the LTFU study, the mean age was 56 years, 85% were female, 90% were white, mean mRSS was 14.8, mean FVC% was 88.2%, and mean HAQ-DI was 0.9. 6 (37.5%) of the ABA group vs 14 (44%) of the non ABA group were also on mycophenolate mofetil or sodium; 2 (12.5%) of the ABA group vs 5 (15.6%) of the non ABA group were on an investigational drug and 7 (43.8%) of the ABA group vs 11 (34.4%) of the non ABA group were on prednisone, with a mean dose of 9.4 mg vs 5.6 mg, respectively. 2 patients on ABA developed serious adverse events (AEs) (new onset pulmonary hypertension and pneumoperitoneum), and 15 AEs of special interest occurred in 6 patients (Table 1). There were 4 deaths during the LTFU – all 4 were in the nonABA group — 1 due to scleroderma renal crisis and 3 due to infections (1 acute respiratory failure due to COVID-19, 1 aspiration pneumonia leading to multi-organ failure, and 1 not specified). 2 (12.5%) patients on ABA vs 6 (18.8%) not on ABA had new or worsening organ involvement or required stem cell transplant (Table 2). The change in the efficacy endpoints from the end of the OLE are shown in Table 3. The lung function improved in the ABA group (mean [SD] FVC% increase of 3.5 [12.7]) while there was a decline in mean FVC% in the non ABA group (-3.9 [12.6]), p=0.07.
Conclusion: In patients with dcSSc, ABA continues to be well tolerated. There are expected AEs including infections and non-melanoma cancers. Patients in the LTFU continue to show improvement in their mRSS and HAQ-DI. The FVC changes in those on ABA vs non ABA may be clinically relevant but further analysis are needed regarding the effect of concomitant immunosuppressive therapies.
.jpg) Table 1: Serious adverse events and adverse events of special interest from patients on abatacept
Table 1: Serious adverse events and adverse events of special interest from patients on abatacept
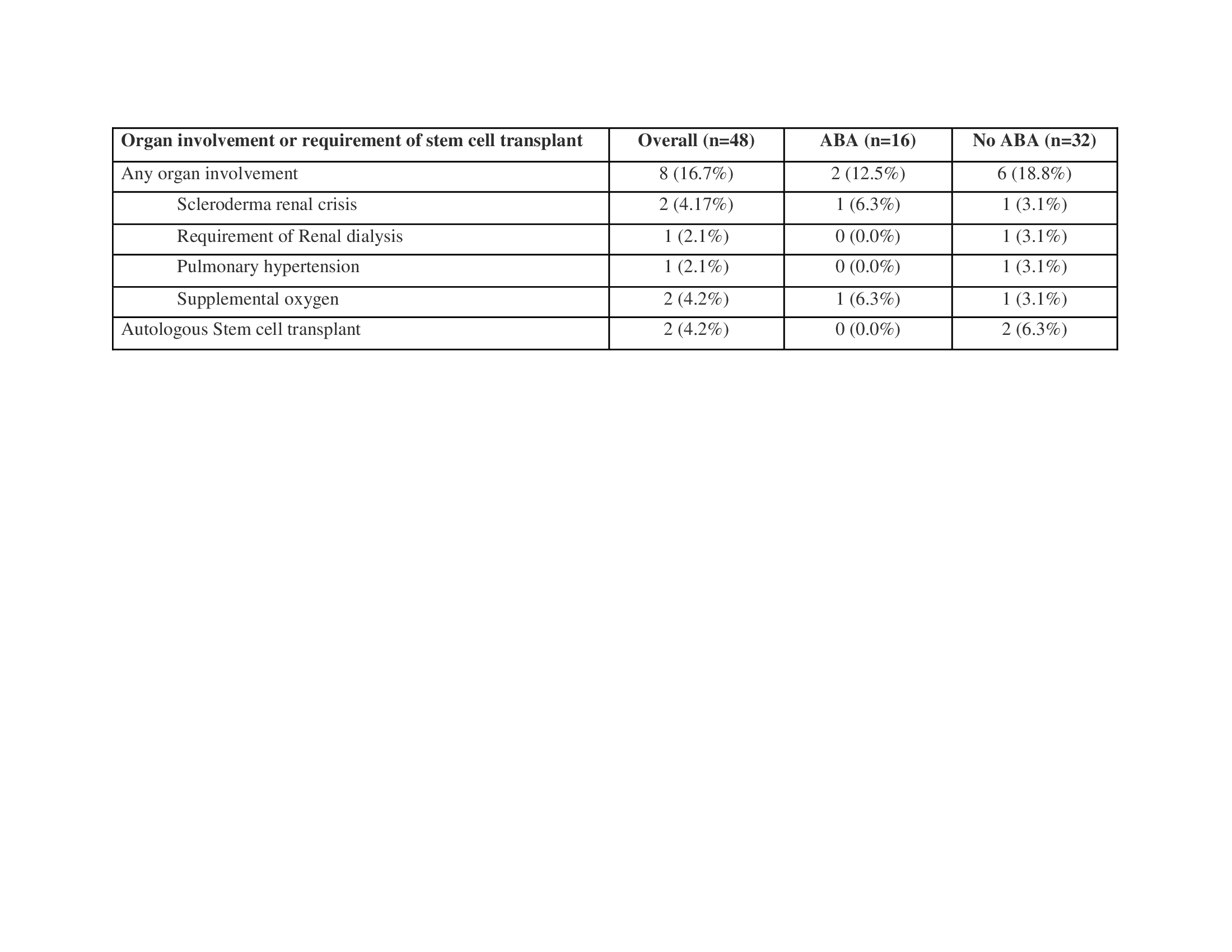 Table 2: Organ involvement during ASSET LTFU
Table 2: Organ involvement during ASSET LTFU
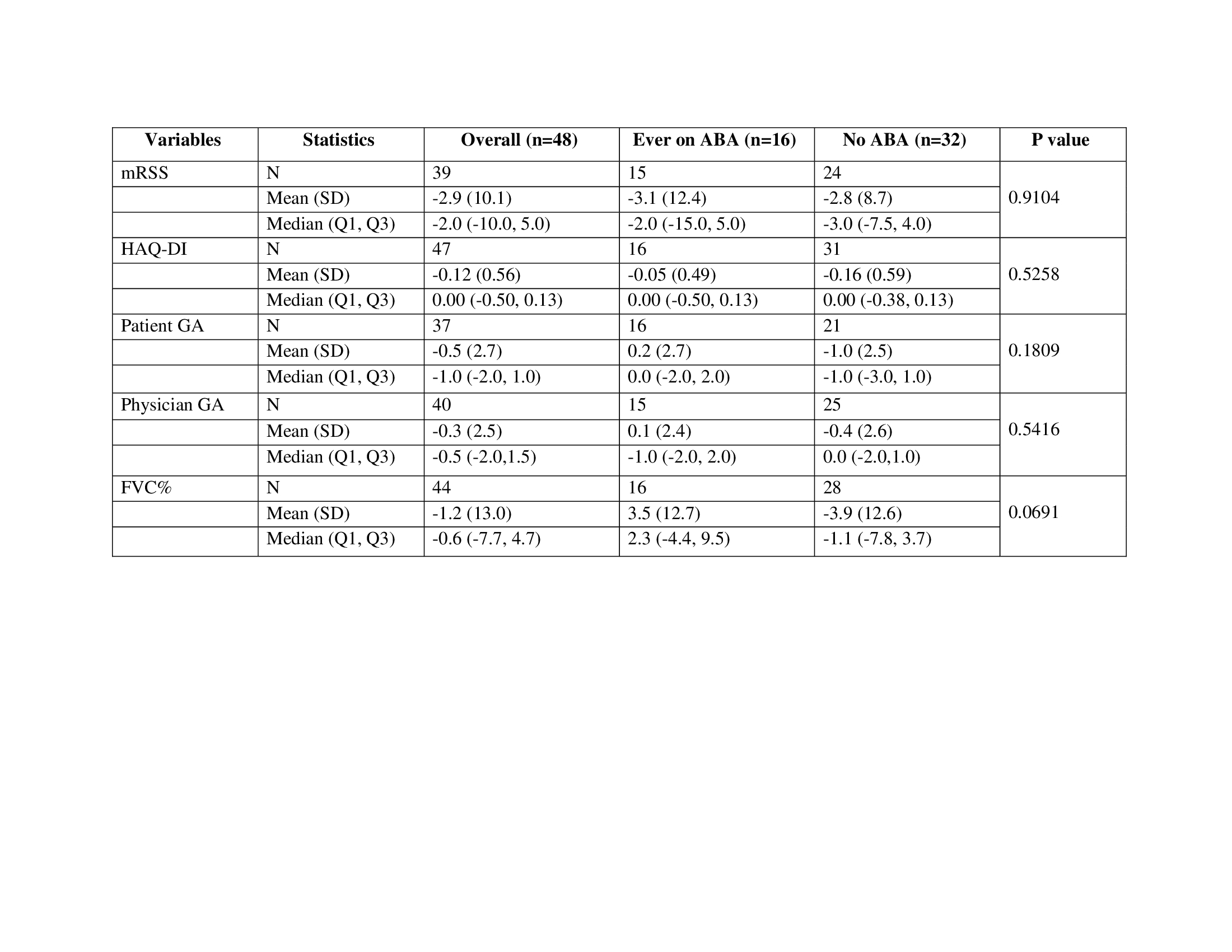 Table 3: Change in the endpoints in participants who were on abatacept and participants who were not on abatacept during the ASSET LTFU
Table 3: Change in the endpoints in participants who were on abatacept and participants who were not on abatacept during the ASSET LTFU
Disclosures: N. Sarsam, None; S. Johnson, None; L. Chung, Kyverna, Mitsubishi Tanabe, Eicos, Boehringer-Ingelheim, Jasper, Genentech; S. Kafaja, None; J. Pope, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Eli Lilly, merk, Roche, Seattle Genetics, UCB, Actelion, Amgen, Bayer, Eicos Sciences, Emerald, Gilead, Janssen, Novartis, Pfizer, Sandoz, Sanofi, Boehringer Ingelheim; R. Domsic, None; M. Mayes, Actelion Pharma, Mitsubishi-Tanabe, Boehringer Ingelheim, EICOS, Horizon Pharma, Prometheus, Corbus, Medtelligence; N. Sandorfi, None; V. Steen, None; F. Castelino, Boehringer-Ingelheim; F. Hant, None; S. Huang, None; D. Khanna, Boehringer Ingelheim, Genentech, Prometheus, Horizon, Chemomab, Talaris, Gesynta, Amgen, Acceleron, Actelion, Bayer, CSL Behring, Paracrine Cell Therapy, Mitsubishi Tanabe, Theraly, Eicos Sciences.
Background/Purpose: Abatacept (ABA) is a cytotoxic T-lymphocyte-associated molecule-4 immunoglobulin fusion protein which has been shown to induce regression of dermal fibrosis in patients with systemic sclerosis (SSc). We previously conducted a 12-month double-blind phase II trial with either ABA or placebo1 followed by a 6-month open-label extension (OLE) with ABA2. This ongoing long term follow up study (LTFU) assessed longer term outcomes, including safety and efficacy of the use of weekly 125 mg subcutaneous ABA in dcSSc, with insights into trends in skin score, functional limitations, and internal organ involvement.
Methods: The current study is ongoing at 11 US and Canadian sites. Inclusion criteria included any participants from the ASSET trial who had participated in the OLE. Each participant signed a new IRB approved consent form. Participants were not required to be on ABA and could be on other immunosuppressive therapies, including investigational agents. The study included two collection time points per year. The outcomes included safety of ABA and change in mRSS (ΔmRSS), ΔHAQ-DI, Δ patient and physician global assessments, and ΔFVC% from the last available assessment in OLE. Mean and standard deviation were reported for efficacy data, and T-test was performed for the difference between ABA and non ABA groups during the LTFU.
Results: 48 patients consented in the ASSET LTFU (16 continued on ABA and 32 on other medications). 44 (92%) participants are continuing in the LTFU. Median time on ABA since the start of LTFU is 1434 days. At baseline of the LTFU study, the mean age was 56 years, 85% were female, 90% were white, mean mRSS was 14.8, mean FVC% was 88.2%, and mean HAQ-DI was 0.9. 6 (37.5%) of the ABA group vs 14 (44%) of the non ABA group were also on mycophenolate mofetil or sodium; 2 (12.5%) of the ABA group vs 5 (15.6%) of the non ABA group were on an investigational drug and 7 (43.8%) of the ABA group vs 11 (34.4%) of the non ABA group were on prednisone, with a mean dose of 9.4 mg vs 5.6 mg, respectively. 2 patients on ABA developed serious adverse events (AEs) (new onset pulmonary hypertension and pneumoperitoneum), and 15 AEs of special interest occurred in 6 patients (Table 1). There were 4 deaths during the LTFU – all 4 were in the nonABA group — 1 due to scleroderma renal crisis and 3 due to infections (1 acute respiratory failure due to COVID-19, 1 aspiration pneumonia leading to multi-organ failure, and 1 not specified). 2 (12.5%) patients on ABA vs 6 (18.8%) not on ABA had new or worsening organ involvement or required stem cell transplant (Table 2). The change in the efficacy endpoints from the end of the OLE are shown in Table 3. The lung function improved in the ABA group (mean [SD] FVC% increase of 3.5 [12.7]) while there was a decline in mean FVC% in the non ABA group (-3.9 [12.6]), p=0.07.
Conclusion: In patients with dcSSc, ABA continues to be well tolerated. There are expected AEs including infections and non-melanoma cancers. Patients in the LTFU continue to show improvement in their mRSS and HAQ-DI. The FVC changes in those on ABA vs non ABA may be clinically relevant but further analysis are needed regarding the effect of concomitant immunosuppressive therapies.
.jpg) Table 1: Serious adverse events and adverse events of special interest from patients on abatacept
Table 1: Serious adverse events and adverse events of special interest from patients on abatacept Table 2: Organ involvement during ASSET LTFU
Table 2: Organ involvement during ASSET LTFU Table 3: Change in the endpoints in participants who were on abatacept and participants who were not on abatacept during the ASSET LTFU
Table 3: Change in the endpoints in participants who were on abatacept and participants who were not on abatacept during the ASSET LTFUDisclosures: N. Sarsam, None; S. Johnson, None; L. Chung, Kyverna, Mitsubishi Tanabe, Eicos, Boehringer-Ingelheim, Jasper, Genentech; S. Kafaja, None; J. Pope, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Eli Lilly, merk, Roche, Seattle Genetics, UCB, Actelion, Amgen, Bayer, Eicos Sciences, Emerald, Gilead, Janssen, Novartis, Pfizer, Sandoz, Sanofi, Boehringer Ingelheim; R. Domsic, None; M. Mayes, Actelion Pharma, Mitsubishi-Tanabe, Boehringer Ingelheim, EICOS, Horizon Pharma, Prometheus, Corbus, Medtelligence; N. Sandorfi, None; V. Steen, None; F. Castelino, Boehringer-Ingelheim; F. Hant, None; S. Huang, None; D. Khanna, Boehringer Ingelheim, Genentech, Prometheus, Horizon, Chemomab, Talaris, Gesynta, Amgen, Acceleron, Actelion, Bayer, CSL Behring, Paracrine Cell Therapy, Mitsubishi Tanabe, Theraly, Eicos Sciences.

