Back
Poster Session C
Rheumatoid arthritis (RA)
Session: (1417–1439) RA – Treatment Poster III
1423: Long-Term Treatment with Golimumab Is a Safe Treatment Option Regardless of Risk Factors in Patients with Rheumatoid Arthritis, Psoriatic Arthritis, and Axial Spondyloarthritis: Results from a Real-World Canadian Setting
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- RA
Regan Arendse, PhD, FRCP, MBChB
Community Rheumatology Care
Saskatoon, SK, Canada
Abstract Poster Presenter(s)
Regan Arendse1, Proton Rahman2, Philip Baer3, Derek Haaland4, Dalton Sholter5, Odalis Asin-Milan6, Meagan Rachich7, Emmanouil Rampakakis8, A. Marilise Marrache9 and Allen J. Lehman10, 1Community Rheumatology Care, Saskatoon, Canada, 2Memorial University, St. John's, NL, Canada, 3Baer Weinberg MPC, Scarborough, ON, Canada, 4The Waterside Clinic, Oro Medonte, ON, Canada, 5University of Alberta, Edmonton, AB, Canada, 6Janssen Canada, Laval, QC, Canada, 7Janssen Inc., Guelph, ON, Canada, 8McGill University, Department of Pediatrics and JSS Medical Research, Montréal, QC, Canada, 9Janssen Inc., Dollard-des-Ormeaux, QC, Canada, 10Janssen Inc., Toronto, ON, Canada
Background/Purpose: Golimumab (GLM), a tumor necrosis factor inhibitor (TNFi), has demonstrated efficacy and a favorable safety profile in inflammatory rheumatic diseases. Recent safety studies with Janus kinase inhibitors (JAKi) sparked new discussions in this therapeutic area, while real-world evidence for GLM continues to support its long-term safety profile1-4. This analysis aimed to describe the risk of major adverse cardiovascular events (MACE), malignancy, and mortality in a large observational cohort of patients (pts) with RA, PsA, or axSpA treated with subcutaneous (SC) GLM. Serious adverse events (SAEs) and serious infections (SIs) were also assessed.
Methods: Post-hoc analysis of the BioTRAC registry, a prospective, multicenter study that collected real-world clinical, laboratory, safety, and patient-reported data in RA, PsA, and axSpA patients treated with GLM, infliximab, or ustekinumab. In the current analysis, only pts treated with SC GLM were included, excluding one pt with prior JAKi treatment. The incidence rates (IR) per 100 patient years (PYs) of AEs of special interest (AEoSI; MACE [defined as cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke], malignancies excluding non-melanoma skin cancer, all-cause death, SAEs, SIs), irrespective of causality to GLM, and time to onset were assessed in subgroups based on the following factors: < 65 vs. ≥65 years of age; sex; prior TNFi experience; smoking status; baseline (BL) use of methotrexate (MTX) and oral steroids. All analyses were stratified by indication.
Results: 1,231 pts were included, 529 with RA, 281 with PsA, and 421 with axSpA. Table 1 summarizes the BL prevalence of risk factors of interest. Over 1064 (RA), 539 (PsA), and 675 (axSpA) PYs, the IR for MACE were 1.1 (n=12), 0.0, and 0.0 events/100 PYs, respectively, while for malignancies the IRs were 1.4, 0.4, and 1.0/100 PYs. SAE incidence ranged from 7.6/100 PYs in PsA pts to 11.4 in RA pts and that of SIs from 1.3/100 PYs in PsA pts to 2.3 in RA pts. IRs for all-cause mortality were 0.7/100 PYs (n=7), 0.2 (n=1), and 0.3 (n=2) /100 PYs in RA, PsA, and axSpA, respectively. Table 2 summarizes the incidence of AEoSI by presence vs absence of risk factors; although numerical differences were observed (e.g. increased rate of malignancy, mortality, and SI in pts ≥65 vs. < 65 years of age), there were no new safety signal. Higher age was associated with shorter time to MACE in RA pts (Figure); no significant association was observed with sex, prior TNFi experience, smoking, and concomitant MTX or steroid use.
Conclusion: Treatment results with GLM in a real-world diverse population are consistent with the current safety profile. This was irrespective of the presence or absence of the examined risk factors for RA, PsA and axSpA. Higher age was associated with shorter time to MACE in RA pts. GLM remains a safe option for the treatment of rheumatic diseases even in the presence of risk factors.
References:
1. Rajpal A. FDA Update on Safety Issues in the Treatment of Rheumatic Disease. ACR Convergence 2021.
2. Rahman P et al. BMC Rheumatol. 2020 Sep 19;4:46
3. Rahman P et al. BMJ Open. 2020 Aug 13;10(8):e036245
4. Rahman P et al. BMC Rheumatol. 2020 Nov 15;4(1):56
.jpg) Table 1. Baseline Risk Factors of Interest by Indication
Table 1. Baseline Risk Factors of Interest by Indication
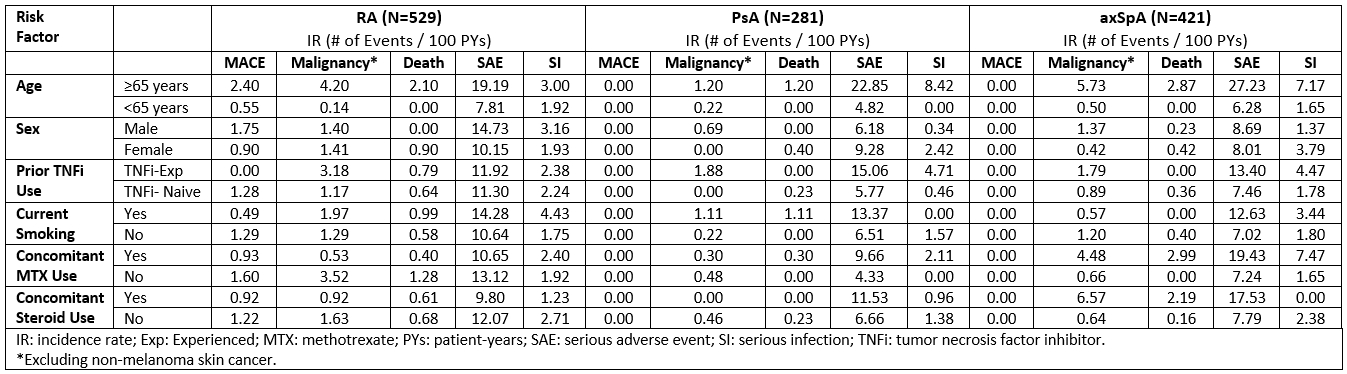 Table 2. Summary of AEs of Special Interest During GLM Treatment by Risk Factor
Table 2. Summary of AEs of Special Interest During GLM Treatment by Risk Factor
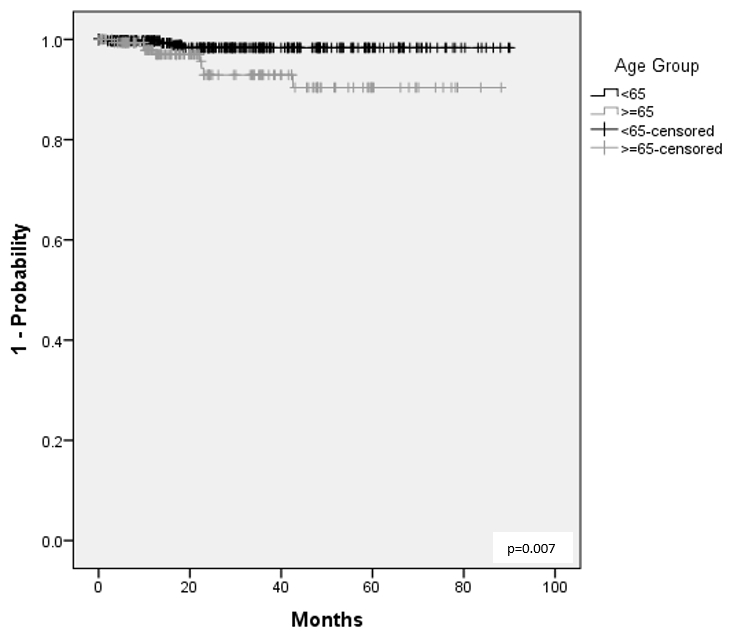 Figure 1. Time to MACE During GLM Treatment by Age Group
Figure 1. Time to MACE During GLM Treatment by Age Group
Disclosures: R. Arendse, None; P. Rahman, Janssen, Novartis, AbbVie, Eli Lilly and Company, Pfizer; P. Baer, Eli Lilly, Pfizer, Novartis, AstraZeneca, GlaxoSmithKlein(GSK), AbbVie, Amgen, Organon, Janssen, Fresenius Kabi, Viatris, Teva, Gilead, Celltrion, Opticann, Innomar, McKesson, JAMP Pharma, Ontario Rheumatology Association, Journal of Rheumatology, Journal of the Canadian Rheumatology Association; D. Haaland, AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, GlaxoSmithKlein(GSK), Janssen, Novartis, Pfizer, Roche, Sanofi Genzyme, Takeda, Merck/MSD, UCB, Celgene, Adiga Life-Sciences, Can-Fite Biopharma, Regeneron, Gilead; D. Sholter, Janssen, Eli Lilly, Novartis, AbbVie/Abbott; O. Asin-Milan, Janssen; M. Rachich, Janssen; E. Rampakakis, Janssen, JSS Medical Research; A. Marrache, Janssen Pharmaceutical Companies of Johnson & Johnson, Johnson & Johnson; A. Lehman, Janssen.
Background/Purpose: Golimumab (GLM), a tumor necrosis factor inhibitor (TNFi), has demonstrated efficacy and a favorable safety profile in inflammatory rheumatic diseases. Recent safety studies with Janus kinase inhibitors (JAKi) sparked new discussions in this therapeutic area, while real-world evidence for GLM continues to support its long-term safety profile1-4. This analysis aimed to describe the risk of major adverse cardiovascular events (MACE), malignancy, and mortality in a large observational cohort of patients (pts) with RA, PsA, or axSpA treated with subcutaneous (SC) GLM. Serious adverse events (SAEs) and serious infections (SIs) were also assessed.
Methods: Post-hoc analysis of the BioTRAC registry, a prospective, multicenter study that collected real-world clinical, laboratory, safety, and patient-reported data in RA, PsA, and axSpA patients treated with GLM, infliximab, or ustekinumab. In the current analysis, only pts treated with SC GLM were included, excluding one pt with prior JAKi treatment. The incidence rates (IR) per 100 patient years (PYs) of AEs of special interest (AEoSI; MACE [defined as cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke], malignancies excluding non-melanoma skin cancer, all-cause death, SAEs, SIs), irrespective of causality to GLM, and time to onset were assessed in subgroups based on the following factors: < 65 vs. ≥65 years of age; sex; prior TNFi experience; smoking status; baseline (BL) use of methotrexate (MTX) and oral steroids. All analyses were stratified by indication.
Results: 1,231 pts were included, 529 with RA, 281 with PsA, and 421 with axSpA. Table 1 summarizes the BL prevalence of risk factors of interest. Over 1064 (RA), 539 (PsA), and 675 (axSpA) PYs, the IR for MACE were 1.1 (n=12), 0.0, and 0.0 events/100 PYs, respectively, while for malignancies the IRs were 1.4, 0.4, and 1.0/100 PYs. SAE incidence ranged from 7.6/100 PYs in PsA pts to 11.4 in RA pts and that of SIs from 1.3/100 PYs in PsA pts to 2.3 in RA pts. IRs for all-cause mortality were 0.7/100 PYs (n=7), 0.2 (n=1), and 0.3 (n=2) /100 PYs in RA, PsA, and axSpA, respectively. Table 2 summarizes the incidence of AEoSI by presence vs absence of risk factors; although numerical differences were observed (e.g. increased rate of malignancy, mortality, and SI in pts ≥65 vs. < 65 years of age), there were no new safety signal. Higher age was associated with shorter time to MACE in RA pts (Figure); no significant association was observed with sex, prior TNFi experience, smoking, and concomitant MTX or steroid use.
Conclusion: Treatment results with GLM in a real-world diverse population are consistent with the current safety profile. This was irrespective of the presence or absence of the examined risk factors for RA, PsA and axSpA. Higher age was associated with shorter time to MACE in RA pts. GLM remains a safe option for the treatment of rheumatic diseases even in the presence of risk factors.
References:
1. Rajpal A. FDA Update on Safety Issues in the Treatment of Rheumatic Disease. ACR Convergence 2021.
2. Rahman P et al. BMC Rheumatol. 2020 Sep 19;4:46
3. Rahman P et al. BMJ Open. 2020 Aug 13;10(8):e036245
4. Rahman P et al. BMC Rheumatol. 2020 Nov 15;4(1):56
.jpg) Table 1. Baseline Risk Factors of Interest by Indication
Table 1. Baseline Risk Factors of Interest by Indication Table 2. Summary of AEs of Special Interest During GLM Treatment by Risk Factor
Table 2. Summary of AEs of Special Interest During GLM Treatment by Risk Factor Figure 1. Time to MACE During GLM Treatment by Age Group
Figure 1. Time to MACE During GLM Treatment by Age GroupDisclosures: R. Arendse, None; P. Rahman, Janssen, Novartis, AbbVie, Eli Lilly and Company, Pfizer; P. Baer, Eli Lilly, Pfizer, Novartis, AstraZeneca, GlaxoSmithKlein(GSK), AbbVie, Amgen, Organon, Janssen, Fresenius Kabi, Viatris, Teva, Gilead, Celltrion, Opticann, Innomar, McKesson, JAMP Pharma, Ontario Rheumatology Association, Journal of Rheumatology, Journal of the Canadian Rheumatology Association; D. Haaland, AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, GlaxoSmithKlein(GSK), Janssen, Novartis, Pfizer, Roche, Sanofi Genzyme, Takeda, Merck/MSD, UCB, Celgene, Adiga Life-Sciences, Can-Fite Biopharma, Regeneron, Gilead; D. Sholter, Janssen, Eli Lilly, Novartis, AbbVie/Abbott; O. Asin-Milan, Janssen; M. Rachich, Janssen; E. Rampakakis, Janssen, JSS Medical Research; A. Marrache, Janssen Pharmaceutical Companies of Johnson & Johnson, Johnson & Johnson; A. Lehman, Janssen.

