Back
Poster Session C
Genetics, genomics and proteomics
Session: (1118–1149) Genetics, Genomics and Proteomics Poster
1137: MHC Class I Polypeptide-Related Sequence a Variant Predicts Real-World Response to Anti-TNF Therapy
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Kristin Wipfler, PhD
FORWARD, The National Databank for Rheumatic Diseases
Omaha, NE, United States
Abstract Poster Presenter(s)
Kristin Wipfler1, Joshua Baker2 and Kaleb Michaud3, 1FORWARD, The National Databank for Rheumatic Diseases, Omaha, NE, 2University of Pennsylvania, Philadelphia, PA, 3University of Nebraska Medical Center, Omaha, NE
Background/Purpose: MHC class I polypeptide-related sequence A (MICA) is a protein involved in the activation of NK and T cells. Variants within the MICA gene have been linked to susceptibility to numerous rheumatic diseases, including RA, PsA, AS, JIA, and SLE, and one variant (rs1051792) has been associated with therapeutic response to TNF inhibitors (TNFi) in patients with RA.1 The objective of this study was to examine the relationships between MICA variants and TNFi response in individuals with RA in a real-world setting.
Methods: Data were provided by adults with RA who provided biosamples and initiated a TNFi during observation in FORWARD, The National Databank for Rheumatic Diseases. These individuals had a baseline observation (prior to medication start) and a follow up observation (after medication start) approximately six months later. Genotyping was performed with the Illumina Infinium Global Screening Array platform, and non-silent common (minor allele frequency > 0.05) MICA variants were included in this analysis (rs1063632, rs1051794, rs1131896, and rs9266825). Change in disease activity following TNFi initiation was measured by minimal clinically important difference (MCID) in the patient activity scale II (PAS-II). The MCID cutoff from baseline to follow up was 0.63 if baseline PAS-II >3.7 and was 0.53 if baseline PAS-II≤3.7. Logistic regression models for achieving MCID were generated for each of the four variants by number of minor alleles present (0, 1, or 2; 0 referent) and were adjusted for baseline PAS-II, age, sex, white race, smoking history, calendar year, RA duration, BMI, glucocorticoid use, and Rheumatic Disease Comorbidity Index (RDCI).
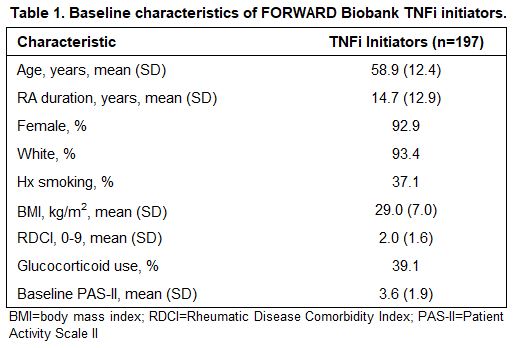
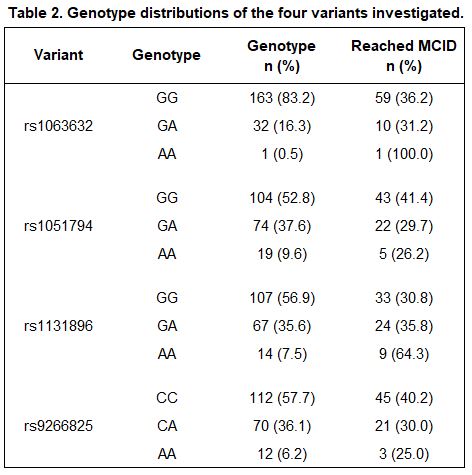
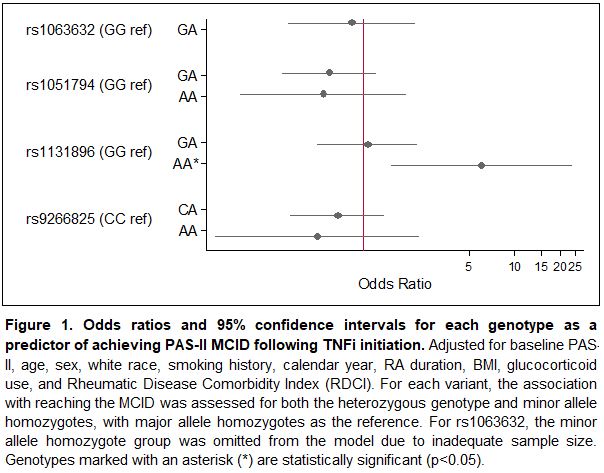
Results: A total of 197 participants met inclusion criteria, 70 of whom (36%) reached the MCID for improved PAS-II following initiation of a TNFi. Baseline characteristics are presented in Table 1 and allele distributions for each variant are presented in Table 2. Of the four variants examined, rs1131896 was significantly associated with improved disease activity following initiation of a TNFi (Figure 1). Individuals who were homozygous for the minor allele at rs1131896 had six times the odds of achieving the MCID for improved PAS-II (OR [95% CI] 6.1 [1.5, 24.1]; p=0.01) compared to those who were homozygous for the major allele. Heterozygous individuals did not have a significant association between their genotype and improved PAS-II scores.
Conclusion: Our results indicate that individuals with RA who are homozygous for the minor allele at MICA rs1131896 are significantly more likely to experience clinically important improvements in disease activity following use of a TNFi. While other MICA variants have been associated with rheumatic diseases and drug response, this is the first study to link rs1131896 with TNF outcomes. This variant may serve as a useful predictor of TNFi response as part of ongoing efforts to improve precision medicine for RA. Future work to confirm this result in additional cohorts and investigate a functional explanation is needed.
1. Iwaszko, M. et al. Association of MICA-129Met/Val polymorphism with clinical outcome of anti-TNF therapy and MICA serum levels in patients with rheumatoid arthritis. Pharmacogenomics J 20, 760-769 (2020).
Disclosures: K. Wipfler, None; J. Baker, Bristol-Myers Squibb(BMS), RediTrex, Pfizer; K. Michaud, None.
Background/Purpose: MHC class I polypeptide-related sequence A (MICA) is a protein involved in the activation of NK and T cells. Variants within the MICA gene have been linked to susceptibility to numerous rheumatic diseases, including RA, PsA, AS, JIA, and SLE, and one variant (rs1051792) has been associated with therapeutic response to TNF inhibitors (TNFi) in patients with RA.1 The objective of this study was to examine the relationships between MICA variants and TNFi response in individuals with RA in a real-world setting.
Methods: Data were provided by adults with RA who provided biosamples and initiated a TNFi during observation in FORWARD, The National Databank for Rheumatic Diseases. These individuals had a baseline observation (prior to medication start) and a follow up observation (after medication start) approximately six months later. Genotyping was performed with the Illumina Infinium Global Screening Array platform, and non-silent common (minor allele frequency > 0.05) MICA variants were included in this analysis (rs1063632, rs1051794, rs1131896, and rs9266825). Change in disease activity following TNFi initiation was measured by minimal clinically important difference (MCID) in the patient activity scale II (PAS-II). The MCID cutoff from baseline to follow up was 0.63 if baseline PAS-II >3.7 and was 0.53 if baseline PAS-II≤3.7. Logistic regression models for achieving MCID were generated for each of the four variants by number of minor alleles present (0, 1, or 2; 0 referent) and were adjusted for baseline PAS-II, age, sex, white race, smoking history, calendar year, RA duration, BMI, glucocorticoid use, and Rheumatic Disease Comorbidity Index (RDCI).
Results: A total of 197 participants met inclusion criteria, 70 of whom (36%) reached the MCID for improved PAS-II following initiation of a TNFi. Baseline characteristics are presented in Table 1 and allele distributions for each variant are presented in Table 2. Of the four variants examined, rs1131896 was significantly associated with improved disease activity following initiation of a TNFi (Figure 1). Individuals who were homozygous for the minor allele at rs1131896 had six times the odds of achieving the MCID for improved PAS-II (OR [95% CI] 6.1 [1.5, 24.1]; p=0.01) compared to those who were homozygous for the major allele. Heterozygous individuals did not have a significant association between their genotype and improved PAS-II scores.
Conclusion: Our results indicate that individuals with RA who are homozygous for the minor allele at MICA rs1131896 are significantly more likely to experience clinically important improvements in disease activity following use of a TNFi. While other MICA variants have been associated with rheumatic diseases and drug response, this is the first study to link rs1131896 with TNF outcomes. This variant may serve as a useful predictor of TNFi response as part of ongoing efforts to improve precision medicine for RA. Future work to confirm this result in additional cohorts and investigate a functional explanation is needed.
1. Iwaszko, M. et al. Association of MICA-129Met/Val polymorphism with clinical outcome of anti-TNF therapy and MICA serum levels in patients with rheumatoid arthritis. Pharmacogenomics J 20, 760-769 (2020).



Disclosures: K. Wipfler, None; J. Baker, Bristol-Myers Squibb(BMS), RediTrex, Pfizer; K. Michaud, None.

