Back
Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1486–1517) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster III
1510: Modifying Lifestyle Factors May Offer the Potential to Enhance the Outcome of Tumour Necrosis Factor Inhibitors in Axial Spondyloarthritis – Data from 14 European Countries
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- GJ
Gareth Jones, PhD
University of Aberdeen
Aberdeen, United Kingdom
Abstract Poster Presenter(s)
Gareth Jones1, Ovidiu Rotariu1, Brigitte Michelsen2, Bente Glintborg3, Bjorn Gudbjornsson4, Arni Geirsson5, Heikki Relas6, Pia Isomäki7, Jakub Závada8, Karel Pavelka9, Ziga Rotar10, Matija Tomsic10, Michael Nissen11, Adrian Ciurea12, Catalin Codreanu13, Johan Karlsson Wallman14, Eirik Kristianslund15, Simon Rasmussen16, Lykke Ørnbjerg17, Maria José Santos18, Mikkel Østergaard19, Merete L Hetland20 and Gary Macfarlane1, 1University of Aberdeen, Aberdeen, Scotland, United Kingdom, 2Center for treatment of Rheumatic and Musculoskeletal Diseases (REMEDY), Diakonhjemmet Hospital, Oslo, Norway, Kristiansand, Norway, 3Rigshospitalet, Glostrup, Virum, Denmark, 4Centre for Rheumatology Research, University Hospital, Reykjavik, Iceland, 5Department of Rheumatology, Landspitali University Hospital, Reykjavik, Iceland, 6Rheumatology, Inflammation Center, Helsinki University Hospital and University of Helsinki, Helsinki, Finland, 7Centre for Rheumatology, Tampere University Hospital, Tampere, Finland, 8Institute of Rheumatology, Prague, Czech Republic, 9Institute of Rheumatology, Department of Rheumatology, 1st Faculty of Medicine, Charles University, Prague, Czech Republic, Praha, Czech Republic, 10University Medical Centre Ljubljana, Ljubljana, Slovenia, 11Hopitaux Universitaires de Genève, Geneva, Switzerland, 12University Hospital Zurich, Zürich, Switzerland, 13Center for Rheumatic Diseases, Bucharest, Romania, 14Lund University and Skane University Hospital, Hjarup, Sweden, 15Diakonhjemmet Hospital, Division of Rheumatology and Research, Oslo, Norway, 16Copenhagen Center for Arthritis Research (COPECARE), Center for Rheumatology and Spine Diseases, Centre for Head and Orthopaedics, Rigshospitalet, Glostrup, Denmark, 17Copenhagen Center for Arthritis Research, Glostrup, Denmark, 18Hospital Garcia de Orta, Almada, Charneca da Caparica, Portugal, 19Rigshospitalet, University of Copenhagen, Glostrup, Denmark, 20Rigshospitalet, Glostrup, Denmark
Background/Purpose: In axial spondyloarthritis (axSpA) much attention has been focused on pharmacological management, including tumour necrosis factor inhibitors (TNFi), as a means to reducing inflammation, and improving function and quality of life. However studies have demonstrated that even with successful control of inflammation symptoms such as pain and fatigue, which have an important impact on quality of life, can remain. The addition of non-pharmacological therapy, including the modification of adverse lifestyle factors, may offer the potential to enhance treatment outcomes in axial spondyloarthritis (axSpA).
Aim: to quantify the impact of lifestyle factors on TNFi treatment response in axSpA.
Methods: Adult axSpA patients commencing their first TNFi treatment were identified via the European Spondyloarthritis Research Collaboration Network (https://eurospa.eu). Participants were required to have information on lifestyle factors (cigarette smoking, alcohol consumption, and/or BMI) recorded within ±30 days of commencing TNFi (baseline), and have outcome data (BASDAI-50 response) at 12 months. The relationship between lifestyle factors and treatment outcome was assessed using logistic regression, and presented as odds ratios, adjusted for age, sex, country, year of TNFi initiation, disease duration and baseline disease activity (BASDAI). The analysis was then repeated for other response criteria (ASDAS inactive disease, low disease activity, clinically important improvement, and major improvement – namely, ASAS-20, ASAS-40, and ASAS-5/6 improvement).
Results: 16,696 participants were included, from 14 axSpA registries across Europe. 29% were current smokers, 49% current drinkers, 37% were overweight and 21% were obese. Other baseline characteristics are shown in Table 1.
Among smokers, 50% met BASDAI-50 response criteria at 12 months, compared to 54% of non-smokers, an association that remained after adjusting for potential confounders (OR: 0.77; 0.68-0.86). Participants who were obese or overweight were less likely to achieve BASDAI-50, compared to patients of normal weight (44%, 53% and 60% respectively) (OR: 0.53; 0.45-0.63 for obese, and 0.76; 0.66-0.87 for overweight). Similar results were seen for other outcome measures and were robust to additional statistical adjustment for other clinical characteristics. Patients who currently drank alcohol were slightly more likely to respond to treatment (BASDAI-50 45% versus 43%; OR: 1.47; 1.16-1.88) an association that was evident across all outcome measures.
Conclusion: Smoking and high BMI were associated with substantial decreases in the odds of TNFi response. For example: if 100 patients were able to give up smoking when commencing TNFi, an additional eight would achieve BASDAI-50 at 12 months. Rheumatologists should consider appropriate smoking cessation and/or weight management interventions at the time of commencing therapy which may offer the potential to improve outcomes.
The relationship between alcohol consumption and increased response to therapy is unlikely to be causal, and the precise nature of this association warrants further investigation.
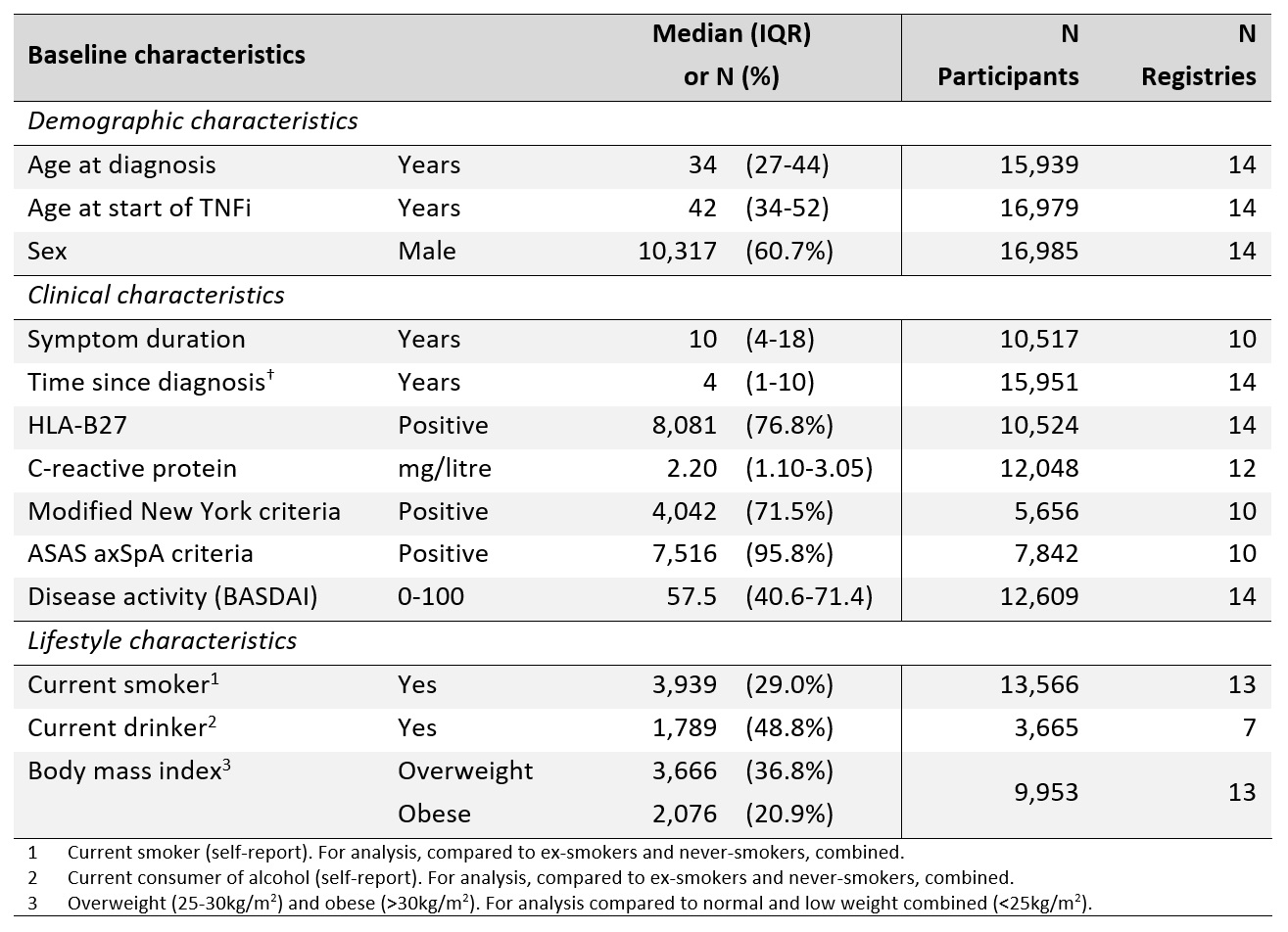 Table 1 – Baseline characteristics
Table 1 – Baseline characteristics
Disclosures: G. Jones, AbbVie/Abbott, Pfizer, UCB, Amgen, GlaxoSmithKlein(GSK), Janssen; O. Rotariu, None; B. Michelsen, Novartis; B. Glintborg, BMS, Pfizer, AbbVie/Abbott; B. Gudbjornsson, Novartis, Amgen; A. Geirsson, None; H. Relas, AbbVie/Abbott, Celgene, Pfizer, UCB, Viatris; P. Isomäki, Pfizer, AbbVie/Abbott, Eli Lilly, Galapagos, Pfizer, Roche, Vifor Pharma; J. Závada, AbbVie/Abbott, Eli Lilly, Sandoz, Novartis, Egis, UCB; K. Pavelka, MSD, Pfizer, Roche, Eli Lilly, Medac, UCB, SOBI, Biogen, Sandoz, Viatris; Z. Rotar, Pfizer, AbbVie/Abbott, Eli Lilly; M. Tomsic, AbbVie/Abbott, Amgen, Biogen, Boehringer-Ingelheim, Eli Lilly, Janssen, Lek-Sandoz, Medis, Merck/MSD, Novartis, Pfizer; M. Nissen, AbbVie/Abbott, Pfizer, Amgen, Novartis, Janssen; A. Ciurea, AbbVie, Novartis, Merck/MSD; C. Codreanu, AbbVie/Abbott, Amgen, AstraZeneca, Boehringer-Ingelheim, Ewopharma, Eli Lilly, Novartis, Pfizer; J. Karlsson Wallman, AbbVie/Abbott, Amgen, Eli Lilly, Novartis, Celgene, Pfizer; E. Kristianslund, None; S. Rasmussen, Novartis; L. Ørnbjerg, Novartis; M. Santos, AbbVie/Abbott, AstraZeneca, pfizer, Novartis, Eli Lilly; M. Østergaard, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, UCB; M. Hetland, Sandoz, Novartis, Pfizer, Eli Lilly, Medac; G. Macfarlane, AbbVie/Abbott, Pfizer, UCB, Amgen, GlaxoSmithKlein(GSK).
Background/Purpose: In axial spondyloarthritis (axSpA) much attention has been focused on pharmacological management, including tumour necrosis factor inhibitors (TNFi), as a means to reducing inflammation, and improving function and quality of life. However studies have demonstrated that even with successful control of inflammation symptoms such as pain and fatigue, which have an important impact on quality of life, can remain. The addition of non-pharmacological therapy, including the modification of adverse lifestyle factors, may offer the potential to enhance treatment outcomes in axial spondyloarthritis (axSpA).
Aim: to quantify the impact of lifestyle factors on TNFi treatment response in axSpA.
Methods: Adult axSpA patients commencing their first TNFi treatment were identified via the European Spondyloarthritis Research Collaboration Network (https://eurospa.eu). Participants were required to have information on lifestyle factors (cigarette smoking, alcohol consumption, and/or BMI) recorded within ±30 days of commencing TNFi (baseline), and have outcome data (BASDAI-50 response) at 12 months. The relationship between lifestyle factors and treatment outcome was assessed using logistic regression, and presented as odds ratios, adjusted for age, sex, country, year of TNFi initiation, disease duration and baseline disease activity (BASDAI). The analysis was then repeated for other response criteria (ASDAS inactive disease, low disease activity, clinically important improvement, and major improvement – namely, ASAS-20, ASAS-40, and ASAS-5/6 improvement).
Results: 16,696 participants were included, from 14 axSpA registries across Europe. 29% were current smokers, 49% current drinkers, 37% were overweight and 21% were obese. Other baseline characteristics are shown in Table 1.
Among smokers, 50% met BASDAI-50 response criteria at 12 months, compared to 54% of non-smokers, an association that remained after adjusting for potential confounders (OR: 0.77; 0.68-0.86). Participants who were obese or overweight were less likely to achieve BASDAI-50, compared to patients of normal weight (44%, 53% and 60% respectively) (OR: 0.53; 0.45-0.63 for obese, and 0.76; 0.66-0.87 for overweight). Similar results were seen for other outcome measures and were robust to additional statistical adjustment for other clinical characteristics. Patients who currently drank alcohol were slightly more likely to respond to treatment (BASDAI-50 45% versus 43%; OR: 1.47; 1.16-1.88) an association that was evident across all outcome measures.
Conclusion: Smoking and high BMI were associated with substantial decreases in the odds of TNFi response. For example: if 100 patients were able to give up smoking when commencing TNFi, an additional eight would achieve BASDAI-50 at 12 months. Rheumatologists should consider appropriate smoking cessation and/or weight management interventions at the time of commencing therapy which may offer the potential to improve outcomes.
The relationship between alcohol consumption and increased response to therapy is unlikely to be causal, and the precise nature of this association warrants further investigation.
 Table 1 – Baseline characteristics
Table 1 – Baseline characteristicsDisclosures: G. Jones, AbbVie/Abbott, Pfizer, UCB, Amgen, GlaxoSmithKlein(GSK), Janssen; O. Rotariu, None; B. Michelsen, Novartis; B. Glintborg, BMS, Pfizer, AbbVie/Abbott; B. Gudbjornsson, Novartis, Amgen; A. Geirsson, None; H. Relas, AbbVie/Abbott, Celgene, Pfizer, UCB, Viatris; P. Isomäki, Pfizer, AbbVie/Abbott, Eli Lilly, Galapagos, Pfizer, Roche, Vifor Pharma; J. Závada, AbbVie/Abbott, Eli Lilly, Sandoz, Novartis, Egis, UCB; K. Pavelka, MSD, Pfizer, Roche, Eli Lilly, Medac, UCB, SOBI, Biogen, Sandoz, Viatris; Z. Rotar, Pfizer, AbbVie/Abbott, Eli Lilly; M. Tomsic, AbbVie/Abbott, Amgen, Biogen, Boehringer-Ingelheim, Eli Lilly, Janssen, Lek-Sandoz, Medis, Merck/MSD, Novartis, Pfizer; M. Nissen, AbbVie/Abbott, Pfizer, Amgen, Novartis, Janssen; A. Ciurea, AbbVie, Novartis, Merck/MSD; C. Codreanu, AbbVie/Abbott, Amgen, AstraZeneca, Boehringer-Ingelheim, Ewopharma, Eli Lilly, Novartis, Pfizer; J. Karlsson Wallman, AbbVie/Abbott, Amgen, Eli Lilly, Novartis, Celgene, Pfizer; E. Kristianslund, None; S. Rasmussen, Novartis; L. Ørnbjerg, Novartis; M. Santos, AbbVie/Abbott, AstraZeneca, pfizer, Novartis, Eli Lilly; M. Østergaard, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, UCB; M. Hetland, Sandoz, Novartis, Pfizer, Eli Lilly, Medac; G. Macfarlane, AbbVie/Abbott, Pfizer, UCB, Amgen, GlaxoSmithKlein(GSK).

