Back
Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1486–1517) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster III
1509: Patient Clusters Identified by Machine Learning from a Pooled Analysis of the Clinical Development Program of Secukinumab in Psoriatic Arthritis, Ankylosing Spondylitis and Psoriatic Arthritis with Axial Manifestations
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- XB
Xenofon Baraliakos, MD
Rheumazentrum Ruhrgebiet Herne
Herne, Germany
Abstract Poster Presenter(s)
Xenofon Baraliakos1, Effie Pournara2, Dafna Gladman3, Philip J Mease4, Samad S Jahandideh2 and Laura Coates5, 1Rheumazentrum Ruhrgebiet Herne, Herne, Germany, 2Novartis Pharma AG, Basel, Switzerland, 3Toronto Western Hospital, Schroeder Arthritis Institute, Toronto, ON, Canada, 4Swedish Medical Center/Providence St. Joseph Health, Seattle, WA, 5Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK, Oxford, England, United Kingdom
Background/Purpose: Universally acceptable clinical and imaging criteria to define axial psoriatic arthritis (PsA) is lacking.1 Machine learning (ML) algorithms can detect patterns from large clinical datasets and identify clusters with potential therapeutic or prognostic significance.2 This post hoc analysis of 10 pooled Phase III trials from the clinical development program of secukinumab in PsA (FUTURE 1–5)3,4, ankylosing spondylitis (AS; MEASURE 1–4)5,6, and patients (pts) with PsA with axial manifestations (MAXIMISE)1, aimed to identify clinical clusters based on pts demographics and baseline clinical indicators to support the characterization of the axial PsA phenotype within the spondyloarthritis (SpA) spectrum.
Methods: Pts demographics (sex, age, BMI) and baseline clinical indicators (swollen and tender joints, Achilles tendon enthesitis, presence of dactylitis, psoriasis [PsO], spinal pain at baseline) were analyzed to identify pts clusters by ML. Finite mixture model methodology was applied to the pooled clinical data of secukinumab-treated pts from 10 clinical trials (FUTURE 1–5, MEASURE 1–4, and MAXIMISE).1, 3-6 This approach assumed multinomial mixture distributions on categorical variables, appropriate for application to a dataset of binary variables. The clustering algorithm was applied repeatedly on different subsamples of the pts to assess clustering robustness and stability.
Results: Overall, 3907 pts were grouped into 8 distinct clusters based on pts demographics and baseline clinical characteristics. PsA pts with axial manifestations from the MAXIMISE trial were overrepresented in clusters 6–8. Pts in cluster 8 were predominantly AS pts, mostly males (63.8%), younger (mean age of 42.9 years), with a mean BMI of 27.3 kg/m2, oligoarthritis and high prevalence of spinal pain. Pts in cluster 6 were commonly female (54%), older (mean age of 48 years), overweight, with notable presence of PsO, and higher articular burden of the knees, shoulders, elbows, and wrists. In comparison, pts in cluster 7 were mostly male (53%), younger (mean age of 47 years) and less overweight with low polyarticular burden and notable tenderness of the joints of the feet, wrists, and hands (Table and Figure). Cluster 1 was predominantly a PsA cluster of older female pts, having high BMI, with polyarticular burden and Achilles tendon enthesitis.
Conclusion: PsA clusters obtained by ML in the pooled dataset of the FUTURE, MEASURE and MAXIMISE trials indicate phenotypical heterogeneity of pts with PsA with axial manifestations and overlapping features across the SpA spectrum. Further studies are needed to explore differences in response to therapy and validate further these clusters to determine phenotypes of pts with SpA.
References
Baraliakos X, et al. Ann Rheum Dis. 2021;80(5):582–590 Pournara E, et al. RMD Open. 2021;7:e00184 Mease PJ, et al. N Engl J Med. 2015 Oct;373(14):1329–39 McInnes IB, et al. Lancet. 2015 Sep 19;386(9999):1137–46 Baeten D, et al. N Engl J Med. 2015 Dec 24;373(26):2534–48 Pavelka K, et al. Arthritis Res Ther. 2017 Dec 22;19(1):285
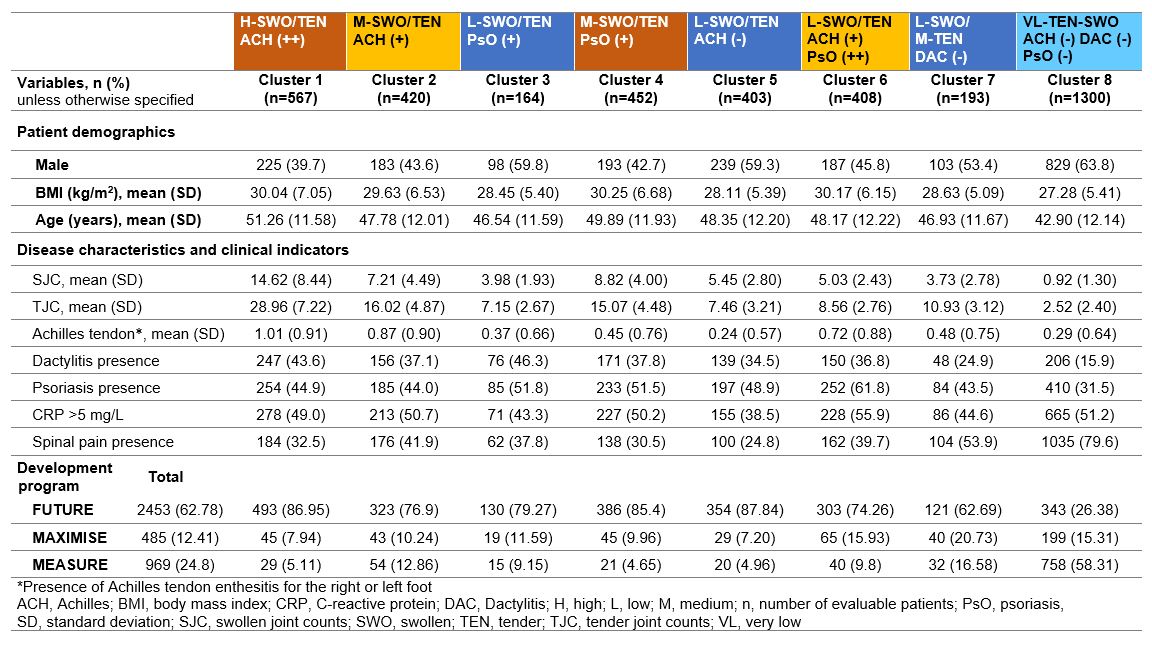 Table. Summary statistics and trial tabulation
Table. Summary statistics and trial tabulation
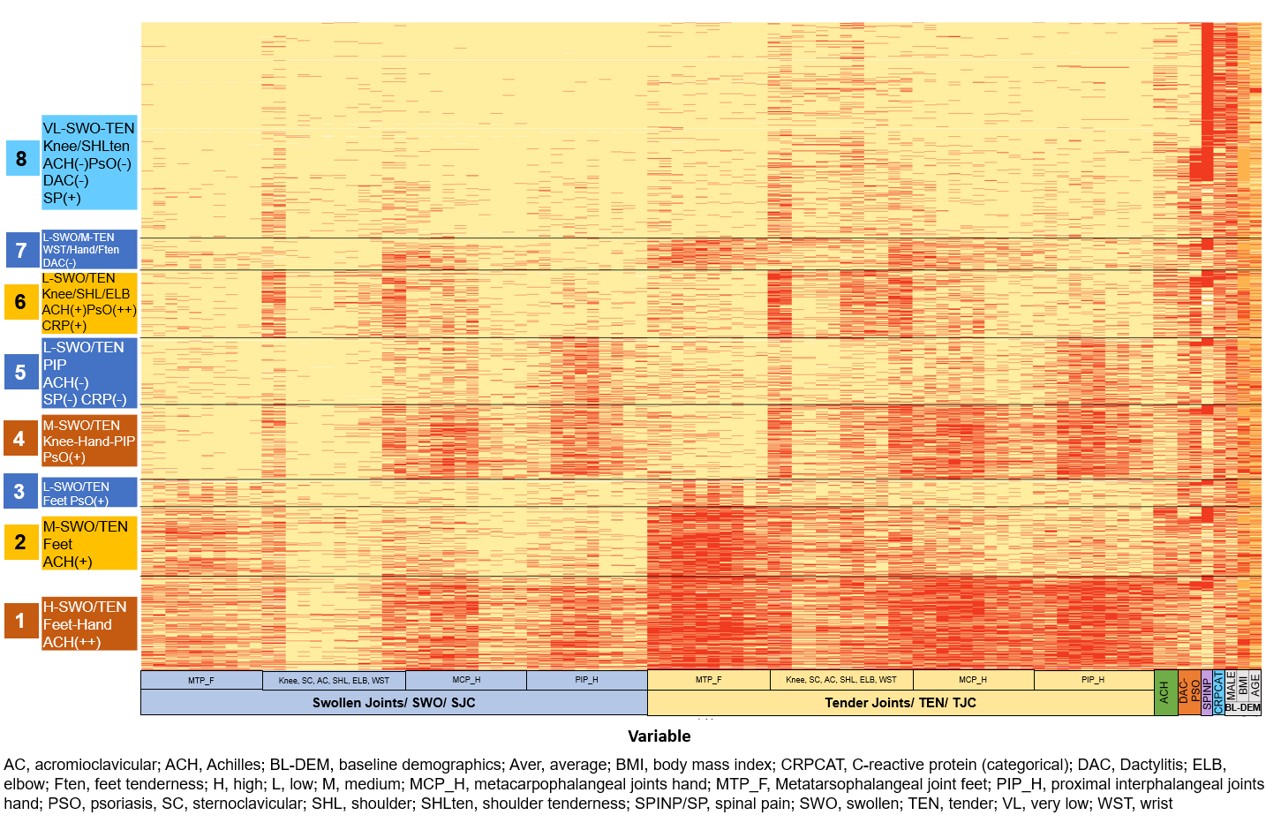 Figure. Heat map of baseline clusters from FUTURE, MEASURE, and MAXIMISE studies
Figure. Heat map of baseline clusters from FUTURE, MEASURE, and MAXIMISE studies
Disclosures: X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi; E. Pournara, Novartis; D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; P. Mease, AbbVie, Amgen, Janssen, Novartis, Pfizer Inc, UCB, Sun Pharma, Eli Lilly, Bristol-Myers Squibb(BMS), Celgene, Genentech; S. Jahandideh, Novartis; L. Coates, AbbVie, Amgen, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, Gilead, Galapagos, Janssen, Medac, Novartis, Pfizer, UCB, Celgene, Biogen, Moonlake, GlaxoSmithKlein (GSK).
Background/Purpose: Universally acceptable clinical and imaging criteria to define axial psoriatic arthritis (PsA) is lacking.1 Machine learning (ML) algorithms can detect patterns from large clinical datasets and identify clusters with potential therapeutic or prognostic significance.2 This post hoc analysis of 10 pooled Phase III trials from the clinical development program of secukinumab in PsA (FUTURE 1–5)3,4, ankylosing spondylitis (AS; MEASURE 1–4)5,6, and patients (pts) with PsA with axial manifestations (MAXIMISE)1, aimed to identify clinical clusters based on pts demographics and baseline clinical indicators to support the characterization of the axial PsA phenotype within the spondyloarthritis (SpA) spectrum.
Methods: Pts demographics (sex, age, BMI) and baseline clinical indicators (swollen and tender joints, Achilles tendon enthesitis, presence of dactylitis, psoriasis [PsO], spinal pain at baseline) were analyzed to identify pts clusters by ML. Finite mixture model methodology was applied to the pooled clinical data of secukinumab-treated pts from 10 clinical trials (FUTURE 1–5, MEASURE 1–4, and MAXIMISE).1, 3-6 This approach assumed multinomial mixture distributions on categorical variables, appropriate for application to a dataset of binary variables. The clustering algorithm was applied repeatedly on different subsamples of the pts to assess clustering robustness and stability.
Results: Overall, 3907 pts were grouped into 8 distinct clusters based on pts demographics and baseline clinical characteristics. PsA pts with axial manifestations from the MAXIMISE trial were overrepresented in clusters 6–8. Pts in cluster 8 were predominantly AS pts, mostly males (63.8%), younger (mean age of 42.9 years), with a mean BMI of 27.3 kg/m2, oligoarthritis and high prevalence of spinal pain. Pts in cluster 6 were commonly female (54%), older (mean age of 48 years), overweight, with notable presence of PsO, and higher articular burden of the knees, shoulders, elbows, and wrists. In comparison, pts in cluster 7 were mostly male (53%), younger (mean age of 47 years) and less overweight with low polyarticular burden and notable tenderness of the joints of the feet, wrists, and hands (Table and Figure). Cluster 1 was predominantly a PsA cluster of older female pts, having high BMI, with polyarticular burden and Achilles tendon enthesitis.
Conclusion: PsA clusters obtained by ML in the pooled dataset of the FUTURE, MEASURE and MAXIMISE trials indicate phenotypical heterogeneity of pts with PsA with axial manifestations and overlapping features across the SpA spectrum. Further studies are needed to explore differences in response to therapy and validate further these clusters to determine phenotypes of pts with SpA.
References
 Table. Summary statistics and trial tabulation
Table. Summary statistics and trial tabulation Figure. Heat map of baseline clusters from FUTURE, MEASURE, and MAXIMISE studies
Figure. Heat map of baseline clusters from FUTURE, MEASURE, and MAXIMISE studiesDisclosures: X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi; E. Pournara, Novartis; D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; P. Mease, AbbVie, Amgen, Janssen, Novartis, Pfizer Inc, UCB, Sun Pharma, Eli Lilly, Bristol-Myers Squibb(BMS), Celgene, Genentech; S. Jahandideh, Novartis; L. Coates, AbbVie, Amgen, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, Gilead, Galapagos, Janssen, Medac, Novartis, Pfizer, UCB, Celgene, Biogen, Moonlake, GlaxoSmithKlein (GSK).

